Chapter 1. Mole Balances
The first step to knowledge is to know that we are ignorant.
—Socrates (470–399 B.C.)
The Wide Wild World of Chemical Reaction Engineering
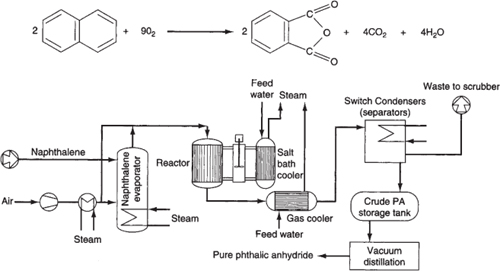
Chemical kinetics is the study of chemical reaction rates and reaction mechanisms. The study of chemical reaction engineering (CRE) combines the study of chemical kinetics with the reactors in which the reactions occur. Chemical kinetics and reactor design are at the heart of producing almost all industrial chemicals, such as the manufacture of phthalic anhydride shown in Figure 1-1. It is primarily a knowledge of chemical kinetics and reactor design that distinguishes the chemical engineer from other engineers. The selection of a reaction system that operates in the safest and most efficient manner can be the key to the economic success or failure of a chemical plant. For example, if a reaction system produces a large amount of undesirable product, subsequent purification and separation of the desired product could make the entire process economically unfeasible.
Figure 1-1. Manufacture of phthalic anhydride.
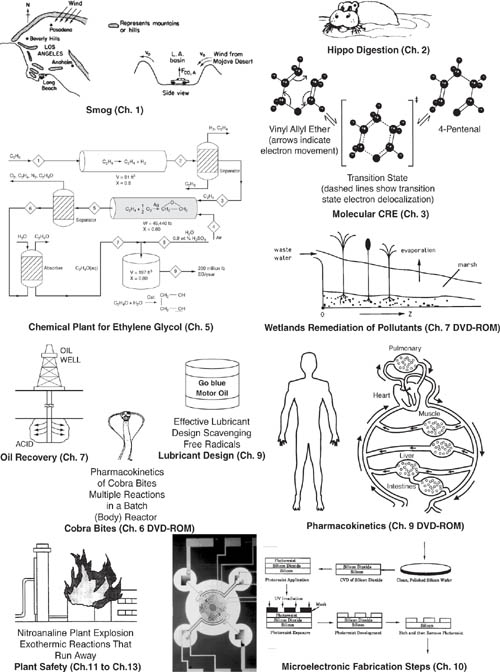
The Chemical Reaction Engineering (CRE) principles learned here can also be applied in many areas, such as waste treatment, microelectronics, nanoparticles, and living systems, in addition to the more traditional areas of the manufacture of chemicals and pharmaceuticals. Some of the examples that illustrate the wide application of CRE principles in this book are shown in Figure 1-2. These examples include modeling smog in the L.A. basin (Chapter 1), the digestive system of a hippopotamus (Chapter 2 DVD-ROM), and molecular CRE (Chapter 3). Also shown are the manufacture of ethylene glycol (antifreeze), where three of the most common types of industrial reactors are used (Chapters 5 and 6), and the use of wetlands to degrade toxic chemicals (Chapter 7 DVD-ROM). Other examples shown are the solid-liquid kinetics of acid-rock interactions to improve oil recovery (Chapter 7); pharmacokinetics of cobra bites (Chapter 8 Web Module); free radical scavengers used in the design of motor oils (Chapter 9); enzyme kinetics (Chapter 9) and drug delivery-pharmacokinetics (Chapter 9 DVD-ROM); heat effects, runaway reactions, and plant safety (Chapters 11 through 13); increasing the octane number of gasoline and the manufacture of computer chips (Chapter 10).
Figure 1-2. The wide world of CRE applications.
1.1 The Rate of Reaction, –rA
The rate of reaction tells us how fast a number of moles of one chemical species are being consumed to form another chemical species. The term chemical species refers to any chemical component or element with a given identity. The identity of a chemical species is determined by the kind, number, and configuration of that species’ atoms. For example, the species para-xylene is made up of a fixed number of specific atoms in a definite molecular arrangement or configuration. The structure shown illustrates the kind, number, and configuration of atoms on a molecular level. Even though two chemical compounds have exactly the same number of atoms of each element, they could still be different species because of different configurations. For example, 2-butene has four carbon atoms and eight hydrogen atoms; however, the atoms in this compound can form two different arrangements.
![]()

As a consequence of the different configurations, these two isomers display different chemical and physical properties. Therefore, we consider them as two different species, even though each has the same number of atoms of each element.
We say that a chemical reaction has taken place when a detectable number of molecules of one or more species have lost their identity and assumed a new form by a change in the kind or number of atoms in the compound and/or by a change in structure or configuration of these atoms. In this classical approach to chemical change, it is assumed that the total mass is neither created nor destroyed when a chemical reaction occurs. The mass referred to is the total collective mass of all the different species in the system. However, when considering the individual species involved in a particular reaction, we do speak of the rate of disappearance of mass of a particular species. The rate of disappearance of a species, say species A, is the number of A molecules that lose their chemical identity per unit time per unit volume through the breaking and subsequent re-forming of chemical bonds during the course of the reaction. In order for a particular species to “appear” in the system, some prescribed fraction of another species must lose its chemical identity.
There are three basic ways a species may lose its chemical identity: decomposition, combination, and isomerization. In decomposition, the molecule loses its identity by being broken down into smaller molecules, atoms, or atom fragments. For example, if benzene and propylene are formed from a cumene molecule,

the cumene molecule has lost its identity (i.e., disappeared) by breaking its bonds to form these molecules. A second way that a molecule may lose its species identity is through combination with another molecule or atom. In the above reaction, the propylene molecule would lose its species identity if the reaction were carried out in the reverse direction, so that it combined with benzene to form cumene. The third way a species may lose its identity is through isomerization, such as the reaction

Here, although the molecule neither adds other molecules to itself nor breaks into smaller molecules, it still loses its identity through a change in configuration.
To summarize this point, we say that a given number of molecules (i.e., moles) of a particular chemical species have reacted or disappeared when the molecules have lost their chemical identity.
The rate at which a given chemical reaction proceeds can be expressed in several ways. To illustrate, consider the reaction of chlorobenzene and chloral to produce the banned insecticide DDT (dichlorodiphenyl-trichloroethane) in the presence of fuming sulfuric acid.
![]()
Letting the symbol A represent chloral, B be chlorobenzene, C be DDT, and D be H2O we obtain
![]()
The numerical value of the rate of disappearance of reactant A, –rA, is a positive number.
The rate of reaction, –rA, is the number of moles of A (e.g., chloral) reacting (disappearing) per unit time per unit volume (mol/dm3·s).
Analysis: The purpose of this example is to better understand the convention for the rate of reaction. The symbol rj is the rate of formation (generation) of species j. If species j is a reactant, the numerical value of rj will be a negative number. If species j is a product, then rj will be a positive number. The rate of reaction, –rA, is the rate of disappearance of reactant A and must be a positive number. A mnemonic relationship to help remember how to obtain relative rates of reaction of A to B, etc., is given by equation (3-1) on page 75.
The convention
–rA = 10 mol A/m3·s
rA = –10 mol A/m3·s
–rB = 20 mol B/m3·s
rB = –20 mol B/m3·s
rC = 10 mol C/m3·s
In Chapter 3, we will delineate the prescribed relationship between the rate of formation of one species, rj (e.g., DDT[C]), and the rate of disappearance of another species, – ri (e.g., chlorobenzene [B]), in a chemical reaction.
Heterogeneous reactions involve more than one phase. In heterogeneous reaction systems, the rate of reaction is usually expressed in measures other than volume, such as reaction surface area or catalyst weight. For a gas-solid catalytic reaction, the gas molecules must interact with the solid catalyst surface for the reaction to take place, as described in Chapter 10.
The dimensions of this heterogeneous reaction rate, ![]() (prime), are the number of moles of A reacting per unit time per unit mass of catalyst (mol/s·g catalyst).
(prime), are the number of moles of A reacting per unit time per unit mass of catalyst (mol/s·g catalyst).
Most of the introductory discussions on chemical reaction engineering in this book focus on homogeneous systems, in which case we simply say that rj is the rate of formation of species j per unit volume. It is the number of moles of species j generated per unit volume per unit time.
We can say four things about the reaction rate rj. The reaction rate law for rj is
• The rate of formation of species j (mole/time/volume)
• An algebraic equation
• Independent of the type of reactor (e.g., batch or continuous flow) in which the reaction is carried out
• Solely a function of the properties of the reacting materials and reaction conditions (e.g., species concentration, temperature, pressure, or type of catalyst, if any) at a point in the system
However, because the properties and reaction conditions of the reacting materials may vary with position in a chemical reactor, rj can in turn be a function of position and can vary from point to point in the system.
The chemical reaction rate law is essentially an algebraic equation involving concentration, not a differential equation.1 For example, the algebraic form of the rate law for –rA for the reaction
![]()
may be a linear function of concentration,
![]()
or, as shown in Chapter 3, it may be some other algebraic function of concentration, such as
![]()
or
![]()
For a given reaction, the particular concentration dependence that the rate law follows (i.e., –rA = kCA or ![]() or ...) must be determined from experimental observation. Equation (1-2) states that the rate of disappearance of A is equal to a rate constant k (which is a function of temperature) times the square of the concentration of A. As noted earlier, by convention, rA is the rate of formation of A; consequently, –rA is the rate of disappearance of A. Throughout this book, the phrase rate of generation means exactly the same as the phrase rate of formation, and these phrases are used interchangeably.
or ...) must be determined from experimental observation. Equation (1-2) states that the rate of disappearance of A is equal to a rate constant k (which is a function of temperature) times the square of the concentration of A. As noted earlier, by convention, rA is the rate of formation of A; consequently, –rA is the rate of disappearance of A. Throughout this book, the phrase rate of generation means exactly the same as the phrase rate of formation, and these phrases are used interchangeably.
1.2 The General Mole Balance Equation
To perform a mole balance on any system, the system boundaries must first be specified. The volume enclosed by these boundaries is referred to as the system volume. We shall perform a mole balance on species j in a system volume, where species j represents the particular chemical species of interest, such as water or NaOH (Figure 1-3).
Figure 1-3. Mole balance on species j in a system volume, V.
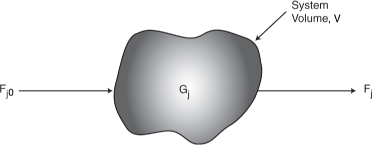
A mole balance on species j at any instant in time, t, yields the following equation:
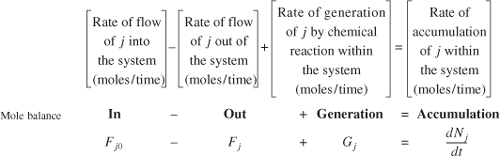
where Nj represents the number of moles of species j in the system at time t. If all the system variables (e.g., temperature, catalytic activity, and concentration of the chemical species) are spatially uniform throughout the system volume, the rate of generation of species j, Gj, is just the product of the reaction volume, V, and the rate of formation of species j, rj.

Now suppose that the rate of formation of species j for the reaction varies with position in the system volume. That is, it has a value rj1 at location 1, which is surrounded by a small volume, ΔV1, within which the rate is uniform: similarly, the reaction rate has a value rj2 at location 2 and an associated volume, ΔV2, and so on (Figure 1-4).
Figure 1-4. Dividing up the system volume, V.

The rate of generation, ΔGj1, in terms of rj1 and subvolume ΔV1, is
ΔGj1 = rj1 ΔV1
Similar expressions can be written for ΔGj2 and the other system subvolumes, ΔVi. The total rate of generation within the system volume is the sum of all the rates of generation in each of the subvolumes. If the total system volume is divided into M subvolumes, the total rate of generation is
![]()
By taking the appropriate limits (i.e., let M → ∞ and ΔV → 0) and making use of the definition of an integral, we can rewrite the foregoing equation in the form
![]()
From this equation we see that rj will be an indirect function of position, since the properties of the reacting materials and reaction conditions (e.g., concentration, temperature) can have different values at different locations in the reactor volume.
We now replace Gj in Equation (1-3)
![]()
by its integral form to yield a form of the general mole balance equation for any chemical species j that is entering, leaving, reacting, and/or accumulating within any system volume V.

From this general mole balance equation, we can develop the design equations for the various types of industrial reactors: batch, semibatch, and continuous-flow. Upon evaluation of these equations, we can determine the time (batch) or reactor volume (continuous-flow) necessary to convert a specified amount of the reactants into products.
1.3 Batch Reactors (BRs)
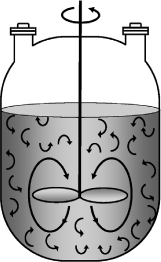
A batch reactor is used for small-scale operation, for testing new processes that have not been fully developed, for the manufacture of expensive products, and for processes that are difficult to convert to continuous operations. The reactor can be charged (i.e., filled) through the holes at the top (see Figure 1-5(a)). The batch reactor has the advantage of high conversions that can be obtained by leaving the reactant in the reactor for long periods of time, but it also has the disadvantages of high labor costs per batch, the variability of products from batch to batch, and the difficulty of large-scale production (see Professional Reference Shelf [PRS] on the DVD-ROM and Web).

Figure 1-5(a). Simple batch homogeneous reactor.

Excerpted by special permission from Chem. Eng., 63(10), 211 (Oct. 1956). Copyright 1956 by McGraw-Hill, Inc., New York, NY 10020.
Figure 1-5(b). Batch reactor mixing patterns. Further descriptions and photos of the batch reactors can be found in both the Visual Encyclopedia of Equipment and in the Professional Reference Shelf on the DVD-ROM.
A batch reactor has neither inflow nor outflow of reactants or products while the reaction is being carried out: Fj0 = Fj = 0. The resulting general mole balance on species j is
![]()
If the reaction mixture is perfectly mixed (Figure 1-5(b)) so that there is no variation in the rate of reaction throughout the reactor volume, we can take rj out of the integral, integrate, and write the mole balance in the form

Let’s consider the isomerization of species A in a batch reactor
![]()

As the reaction proceeds, the number of moles of A decreases and the number of moles of B increases, as shown in Figure 1-6.
Figure 1-6. Mole-time trajectories.
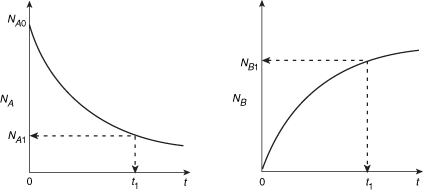
We might ask what time, t1, is necessary to reduce the initial number of moles from NA0 to a final desired number NA1. Applying Equation (1-5) to the isomerization
![]()
rearranging,
![]()
and integrating with limits that at t = 0, then NA = NA0, and at t = t1, then NA = NA1, we obtain
![]()
This equation is the integral form of the mole balance on a batch reactor. It gives the time, t1, necessary to reduce the number of moles from NA0 to NA1 and also to form NB1 moles of B.
1.4 Continuous-Flow Reactors
Continuous flow reactors are almost always operated at steady state. We will consider three types: the continuous-stirred tank reactor (CSTR), the plug flow reactor (PFR), and the packed-bed reactor (PBR). Detailed physical descriptions of these reactors can be found in both the Professional Reference Shelf (PRS) for Chapter 1 and in the Visual Encyclopedia of Equipment on the DVD-ROM.


