1.4.1 Continuous-Stirred Tank Reactor (CSTR)
A type of reactor used commonly in industrial processing is the stirred tank operated continuously (Figure 1-7). It is referred to as the continuous-stirred tank reactor (CSTR) or vat, or backmix reactor, and is used primarily for liquid phase reactions. It is normally operated at steady state and is assumed to be perfectly mixed; consequently, there is no time dependence or position dependence of the temperature, concentration, or reaction rate inside the CSTR. That is, every variable is the same at every point inside the reactor. Because the temperature and concentration are identical everywhere within the reaction vessel, they are the same at the exit point as they are elsewhere in the tank. Thus, the temperature and concentration in the exit stream are modeled as being the same as those inside the reactor. In systems where mixing is highly nonideal, the well-mixed model is inadequate, and we must resort to other modeling techniques, such as residence-time distributions, to obtain meaningful results. This topic of nonideal mixing is discussed in DVD-ROM Chapters DVD13 and DVD14, on the DVD-ROM included with this text, and in Chapters 13 and 14 in the fourth edition of The Elements of Chemical Reaction Engineering (ECRE).
Figure 1-7(a). CSTR/batch reactor.

Courtesy of Pfaudler, Inc.
Figure 1-7(b). CSTR mixing patterns. Also see the Visual Encyclopedia of Equipment on the DVD-ROM.

When the general mole balance equation
![]()
is applied to a CSTR operated at steady state (i.e., conditions do not change with time),
in which there are no spatial variations in the rate of reaction (i.e., perfect mixing),
![]()
it takes the familiar form known as the design equation for a CSTR:
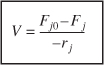

The CSTR design equation gives the reactor volume V necessary to reduce the entering flow rate of species j from Fj0 to the exit flow rate Fj, when species j is disappearing at a rate of –rj. We note that the CSTR is modeled such that the conditions in the exit stream (e.g., concentration, and temperature) are identical to those in the tank. The molar flow rate Fj is just the product of the concentration of species j and the volumetric flow rate υ:

Similarly, for the entrance molar flow rate we have Fj0 = Cj0 · υ0. Consequently, we can substitute for Fj0 and Fj into Equation (1-7) to write a balance on species A as
![]()
The ideal CSTR mole balance equation is an algebraic equation, not a differential equation.
