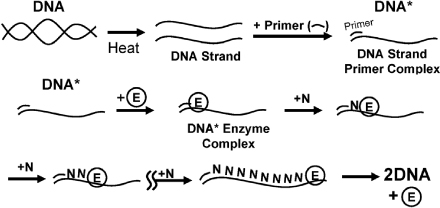
9.2.4 Batch Reactor Calculations for Enzyme Reactions
A mole balance on urea in a batch reactor gives
![]()
Because this reaction is liquid phase, V = V0, the mole balance can be put in the following form:
![]()
The rate law for urea decomposition is
![]()
Substituting Equation (9-31) into Equation (9-30) and then rearranging and integrating, we get

We can write Equation (9-32) in terms of conversion as
Curea = Curea0 (1 = X)

The parameters KM and Vmax can readily be determined from batch reactor data by using the integral method of analysis. Dividing both sides of Equation (9-32) by (tKM/Vmax) and rearranging yields
![]()
We see that KM and Vmax can be determined from the slope and intercept of a plot of (1/t) ln[1/(1 = X)] versus X/t. We could also express the Michaelis–Menten equation in terms of the substrate concentration S:

where S0 is the initial concentration of substrate. In cases similar to Equation (9-33) where there is no possibility of confusion, we shall not bother to enclose the substrate or other species in parentheses to represent concentration [i.e., CS ≡ (S) ≡ S]. The corresponding plot in terms of substrate concentration is shown in Figure 9-7.
Figure 9-7. Evaluating Vmax and KM from batch reactor data.

Example 9-3. Batch Enzymatic Reactors
Calculate the time needed to convert 99% of the urea to ammonia and carbon dioxide in a 0.5-dm3 batch reactor. The initial concentration of urea is 0.1 mol/dm3, and the urease concentration is 0.001 g/dm3. The reaction is to be carried out isothermally at the same temperature at which the data in Table E9-2.2 were obtained.
We can use Equation (9-32),
![]()
where KM = 0.0266 mol/dm3, X = 0.99, and Curea0 = 0.1 mol/dm3, Vmax was 1.33 mol/dm3·s. However, for the conditions in the batch reactor, the enzyme concentration is only 0.001 g/dm3, compared with 5 g/dm3 in Example 9-2. Because Vmax = Et · k3, Vmax for the second enzyme concentration is

Substituting into Equation (9-32)

Analysis: This example shows a straightforward Chapter 5 type calculation of the batch reactor time to achieve a certain conversion X for an enzymatic-reaction with a Michaelis–Menten rate law. This batch reaction time is very short; consequently, a continuous flow reactor would be better suited for this reaction.
Effect of Temperature
The effect of temperature on enzymatic reactions is very complex. If the enzyme structure would remain unchanged as the temperature is increased, the rate would probably follow the Arrhenius temperature dependence. However, as the temperature increases, the enzyme can unfold and/or become denatured and lose its catalytic activity. Consequently, as the temperature increases, the reaction rate, –rS, increases up to a maximum and then decreases as the temperature is increased further. The descending part of this curve is called temperature inactivation or thermal denaturizing.10 Figure 9-8 shows an example of this optimum in enzyme activity.11
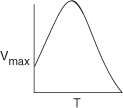
Figure 9-8. Catalytic breakdown rate of H2O2 depending on temperature.

Courtesy of S. Aiba, A. E. Humphrey, and N. F. Mills, Biochemical Engineering, Academic Press (1973).
9.3 Inhibition of Enzyme Reactions
In addition to temperature and solution pH, another factor that greatly influences the rates of enzyme-catalyzed reactions is the presence of an inhibitor. Inhibitors are species that interact with enzymes and render the enzyme ineffective to catalyze its specific reaction. The most dramatic consequences of enzyme inhibition are found in living organisms, where the inhibition of any particular enzyme involved in a primary metabolic pathway will render the entire pathway inoperative, resulting in either serious damage or death of the organism. For example, the inhibition of a single enzyme, cytochrome oxidase, by cyanide will cause the aerobic oxidation process to stop; death occurs in a very few minutes. There are also beneficial inhibitors, such as the ones used in the treatment of leukemia and other neoplastic diseases. Aspirin inhibits the enzyme that catalyzes the synthesis of the module prostaglandin, which is involved in the pain-producing process. Recently the discovery of DDP-4 enzyme inhibitor Januvia has been approved for the treatment of Type 2 diabetes, a disease affecting 240 million people worldwide (see P9-14B).
The three most common types of reversible inhibition occurring in enzymatic reactions are competitive, uncompetitive, and noncompetitive. The enzyme molecule is analogous to a heterogeneous catalytic surface in that it contains active sites. When competitive inhibition occurs, the substrate and inhibitor are usually similar molecules that compete for the same site on the enzyme. Uncompetitive inhibition occurs when the inhibitor deactivates the enzyme-substrate complex, sometimes by attaching itself to both the substrate and enzyme molecules of the complex. Noncompetitive inhibition occurs with enzymes containing at least two different types of sites. The substrate attaches only to one type of site, and the inhibitor attaches only to the other to render the enzyme inactive.