11.3.3 Relating ΔHRx(T),  , and ΔCP
, and ΔCP
Recall that the heat of reaction at temperature T, was given in terms of the enthalpy of each reacting species at temperature T in Equation (11-14), that is
![]()
where the enthalpy of each species is given by
![]()
If we now substitute for the enthalpy of each species, we have
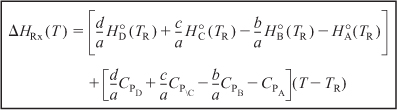
The first term in brackets on the right-hand side of Equation (11-23) is the heat of reaction at the reference temperature TR,

The enthalpies of formation of many compounds, ![]() , are usually tabulated at 25°C and can readily be found in the Handbook of Chemistry and Physics1 and similar handbooks. That is, we can look up the heats of formation at TR, then calculate the heat of reaction at this reference temperature. The heat of combustion (also available in these handbooks) can also be used to determine the enthalpy of formation,
, are usually tabulated at 25°C and can readily be found in the Handbook of Chemistry and Physics1 and similar handbooks. That is, we can look up the heats of formation at TR, then calculate the heat of reaction at this reference temperature. The heat of combustion (also available in these handbooks) can also be used to determine the enthalpy of formation, ![]() , and the method of calculation is described in these handbooks. From these values of the standard heat of formation,
, and the method of calculation is described in these handbooks. From these values of the standard heat of formation, ![]() , we can calculate the heat of reaction at the reference temperature TR using Equation (11-24).
, we can calculate the heat of reaction at the reference temperature TR using Equation (11-24).
The second term in brackets on the right-hand side of Equation (11-23) is the overall change in the heat capacity per mole of A reacted, ΔCP,
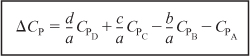
Combining Equations (11-25), (11-24), and (11-23) gives us
![]()
Equation (11-26) gives the heat of reaction at any temperature T in terms of the heat of reaction at a reference temperature (usually 298 K) and the ΔCP term. Techniques for determining the heat of reaction at pressures above atmospheric can be found in Chen.2 For the reaction of hydrogen and nitrogen at 400°C, it was shown that the heat of reaction increased by only 6% as the pressure was raised from 1 atm to 200 atm!
Example 11-2. Heat of Reaction
Calculate the heat of reaction for the synthesis of ammonia from hydrogen and nitrogen at 150°C in kcal/mol of N2 reacted and also in kJ/mol of H2 reacted.
![]()
Calculate the heat of reaction at the reference temperature using the heats of formation of the reacting species obtained from Perry’s Handbook3 or the Handbook of Chemistry and Physics.
The enthalpies of formation at 25°C are
![]()
Note: The heats of formation of all elements (e.g., H2, N2) are zero at 25°C.
To calculate ![]() we take the heats of formation of the products (e.g., NH3) multiplied by their appropriate stoichiometric coefficients (2 for NH3) minus the heats of formation of the reactants (e.g., N2, H2) multiplied by their stoichiometric coefficient (e.g., 3 for H2, 1 of N2).
we take the heats of formation of the products (e.g., NH3) multiplied by their appropriate stoichiometric coefficients (2 for NH3) minus the heats of formation of the reactants (e.g., N2, H2) multiplied by their stoichiometric coefficient (e.g., 3 for H2, 1 of N2).

![]()
The minus sign indicates that the reaction is exothermic. If the heat capacities are constant or if the mean heat capacities over the range 25°C to 150°C are readily available, the determination of ΔHRx at 150°C is quite simple.


(Recall: 1 kcal = 4.184 kJ)
The heat of reaction based on the moles of H2 reacted is

Analysis: This example showed (1) how to calculate the heat of reaction with respect to a given species, given the heats of formation of the reactants and the products, and (2) how to find the heat of reaction with respect to one species, given the heat of reaction with respect to another species in the reaction. We also saw how the heat of reaction changed as we increased the temperature.
Now that we see that we can calculate the heat of reaction at any temperature, let’s substitute Equation (11-22) in terms of ΔHR(TR) and ΔCP [i.e., Equation (11-26)]. The steady-state energy balance is now
![]()
From here on, for the sake of brevity we will let
![]()
unless otherwise specified.
In most systems, the work term, ![]() , can be neglected (note the exception in the California Professional Engineers’ Exam Problem P12-6B at the end of Chapter 12). Neglecting
, can be neglected (note the exception in the California Professional Engineers’ Exam Problem P12-6B at the end of Chapter 12). Neglecting ![]() , the energy balance becomes
, the energy balance becomes
![]()
In almost all of the systems we will study, the reactants will be entering the system at the same temperature; therefore, Ti0 = T0.
We can use Equation (11-28) to relate temperature and conversion and then proceed to evaluate the algorithm described in Example 11-1. However, unless the reaction is carried out adiabatically, Equation (11-28) is still difficult to evaluate because in nonadiabatic reactors, the heat added to or removed from the system varies along the length of the reactor. This problem does not occur in adiabatic reactors, which are frequently found in industry. Therefore, the adiabatic tubular reactor will be analyzed first.
11.4 Adiabatic Operation
Reactions in industry are frequently carried out adiabatically with heating or cooling provided either upstream or downstream. Consequently, analyzing and sizing adiabatic reactors is an important task.
