Coal formation
Abstract:
This chapter discusses coal formation, coal types, and coalification – the progression through the ranks of coal. Many factors affected peat formation – climate, geology, chemistry, types of plants, etc. – and the conditions in the peat swamp affected the decay of plant material that resulted in differences in coal types. Once peat formation ceased and the peat was buried, coal was formed and then progressed through the ranks – from lignite to anthracite, over time and with exposure to pressure and temperature. Descriptions of important coal properties and coal classification standards are given in this chapter with a discussion of the changes that occur with coal rank.
2.1 Introduction
This chapter discusses coal formation, coal types and coalification – the progression through the ranks of coal. Coal was formed from plant material deposited as peat in swampy environments. Many factors affected peat formation – climate, geology, chemistry, types of plants, etc. – and the conditions in the swamp affected the decay of plant material that resulted in differences in coal types. Coal type that covers most banded coals (vitrain, clarain, durain, and fusain) is discussed in this chapter as a prelude to additional discussions on coal macerals in the following chapters.
Once the peat was buried under sediments and cut off from additional plant matter deposition, coal could be formed. An approximate line is drawn between peat and coal. Once a peat is compressed and buried to a point where the moisture content inherent in the formation is less than 75%, it is then known, generally, as coal (ISO, 2005). Coal then progresses through the ranks – from lignite to bituminous to anthracite, over time and with exposure to pressure and temperature. Coal properties change and many show a maximum or minimum in the ‘coking’ coal range – the high volatile A to low volatile bituminous coals. Descriptions of important coal properties and coal classification standards are given in this chapter with a discussion of the changes that occur with coal rank.
2.2 Coal formation
Coal and other fossil fuels contain carbon, the sixth most common element in the universe. Carbon exists in the atmosphere, in water, and in rocks, generally as carbon dioxide. Plants and animals utilize carbon or carbon dioxide in many ways. For example, plants take in carbon dioxide and give off oxygen, while animals breathe oxygen and give off carbon dioxide. Once these plants and animals die and decay, additional carbon dioxide is released into the atmosphere. This global carbon cycle is depicted graphically in Fig. 2.1 (adapted from Radovic and Schobert, 1992). As described by Radovic and Schobert (1992), this cycle can be interrupted by events – floods, earthquakes, volcanic eruptions, mountain formation, etc. – which cause the decaying plants and animals to be buried under sediments.

2.1 Global carbon cycle interrupted for fossil fuel formation. (Source: Adapted from Radovic and Schobert, 1992.)
The buried, accumulated organic matter undergoes further decay by bacteria (aerobic or anaerobic depending on the oxygen availability) and after thousands of years, kerogen forms. With deeper burial and the associated increasing temperature, kerogen breaks into shorter chain carbon molecules. Kerogen from algae and plankton forms crude oil, and with additional time and temperature, it will form gas.
Kerogen from higher plants has more structure and rigidity. The carbon atoms will form rings rather than chains as in oil or gas. This kerogen will eventually form peat after exposure to heat, pressure, and time (10–100 million years). During this process, there are multiple opportunities for minerals to enter the peat and for multiple thin layers to form.
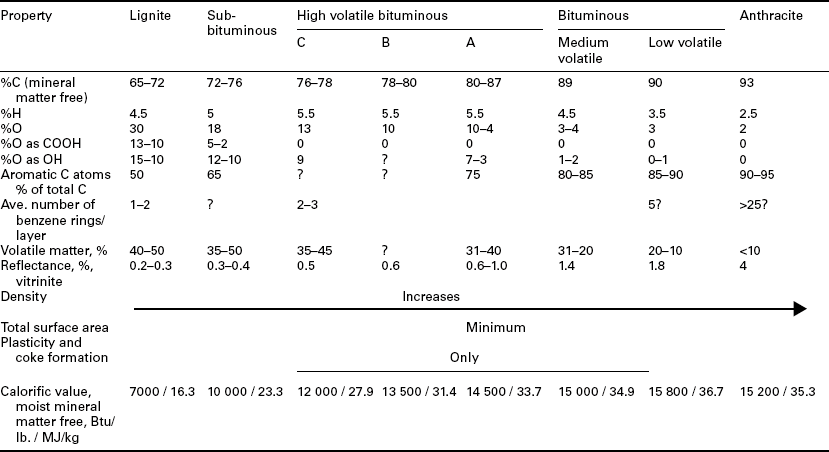
Once plant material deposition ceases, from burial under sediments, for example, and following additional exposure to heat, pressure, and time, the peat will eventually become coal – a designation given when the moisture content inherent in the formation is less than 75% (ISO, 2005). Depending on the combination of heat (from depth of burial or igneous intrusions), pressure (from depth of burial or from mountain-building episodes) and time, coal will progress then through different ranks – from brown coal or lignite through the higher bituminous and anthracite ranks (hard coal). Throughout this ‘coalification’ process, coal chemistry and other properties change. As shown in Table 2.1, Given (1975) has provided some approximate values for coal properties with increasing coal rank – with the names for various coal ranks given as described by the American Society for Testing and Materials (ASTM, 2005). The first changes are physical – compaction of the peat with a loss in moisture. Next, oxygen functional groups are lost, with a corresponding increase in calorific content. Volatile matter yield and hydrogen then show a marked decrease with further coalification. A final stage of coalification can be reached when the coal is subjected to metamorphic activity – the aromatic lamellae are rearranged into a more graphite-like structure. Indeed, graphite can be found in association with some anthracite deposits. A more detailed description of these and other coal properties and the changes with coal rank will be provided in a later section.
This description simplifies the process of ‘coalification’ or the formation of coal and progression through the ranks of coal. It is important to understand coal formation from this simplified perspective to then understand that no two coals are alike. Even coal within a distinct coal seam will vary, based on opportunities for mineral incursions in the peat swamp or exposure to igneous intrusions that apply additional localized heat that can increase coal rank, and result in very different coal properties and chemistry.
2.3 Coal types
The type of plants available for coal formation changed over geological time and with differing climatic conditions. Differing plant types along with differing conditions in the peat swamp led to differences in coal types that today affect their mining, processing, and utilization.
While a detailed description of coal macerals and macroscopic and microscopic coal types will be given in Chapter 3, a brief description is warranted here. It follows the Stopes-Heerlen system of classification for Eastern US and European coals (McClung and Geer, 1979) and covers coals sometimes described as ‘banded’ or ‘humic’ rather than some special coals, ‘sapropelic’, which are noted for their high content of hydrogen and lack stratification (Stach et al., 1975). The ‘banded’ coal types are a result of both the original plant matter and the conditions for decay in the peat swamp. Shiny bands of coal – vitrain – are composed primarily of the maceral vitrinite. Vitrinite is formed from the higher plants, and vitrain bands are found when these layers of organic material were less disturbed by decay or incursions of minerals.
Bands that are still somewhat shiny, but more broken, would be termed clarain. These bands still contain significant amounts of vitrinite, but it will be banded to a greater extent in a hand specimen and even microscopically. The vitrinite will be accompanied by liptinite (or exinite) macerals that formed from plant components that were more resistant to decay, like the more waxy plant material – spores, for example. Some inertinite macerals (from plant material subjected to significant decay, oxidation or fire) can be present.
When most of the shiny banding is gone from a hand specimen, this is termed a durain. Some vitrinite will persist, but inertinite macerals will predominate. If a sample is very friable and ‘dirty’, it may be a fusain – primarily composed of the inertinite macerals, fusinite and semifusinite.
Each of these coal types behave differently in mining and processing, as well as in utilization. The initial plant matter played a role in determining coal type. These different plant materials also had different chemical compositions, and these differences are not lost in the transformation of plant matter into coal. For example, the liptinite (or exinite) macerals contain more volatile matter than the associated vitrinite and fusinite. One property of vitrinite, however, is related specifically to its aromaticity – the percentage of light reflected back from the surface of a polished section of the vitrinite. Aromaticity increases with increasing coal rank (van Krevelen, 1961), and the aromaticity of vitrinite changes most consistently with increasing rank. Therefore, the mean maximum vitrinite reflectance is used often as a measure of coal rank to remove the variable of maceral content from rank determination.
2.4 Differences in coalification history
Conditions that allowed the deposition and burial of plant material for peat formation and for subsequent coal formation have occurred under different climates and with different plant materials, with major coal deposits being formed in every geologic age since the Carboniferous and a few formed in the earlier Devonian and Silurian periods (see summary in Table 2.2). Some have occurred over large basins with limited influx of minerals, especially in the interior of the basin, while other depositional environments allowed mineral incursions to create significant mineral partings in the coal bed and limit seam height. In some cases, especially with some Indian and South African coals, the minerals and coal are mixed in such a fashion as to limit the extent to which the coal can be cleaned economically.
Table 2.2
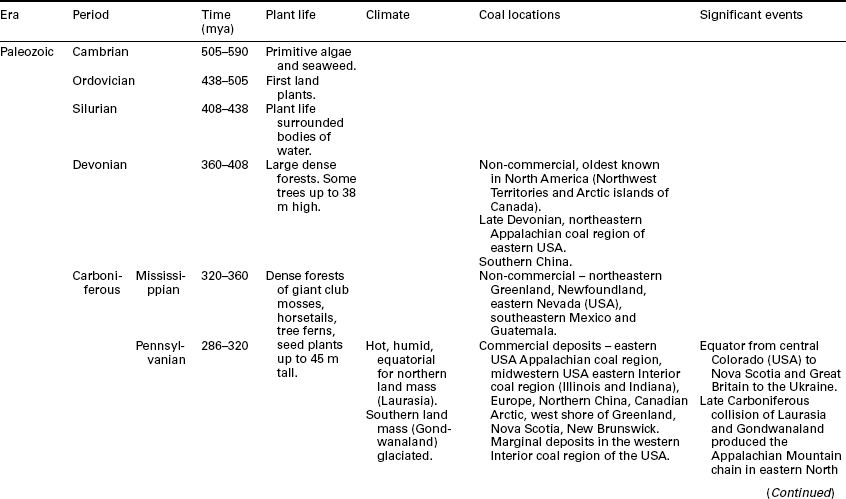


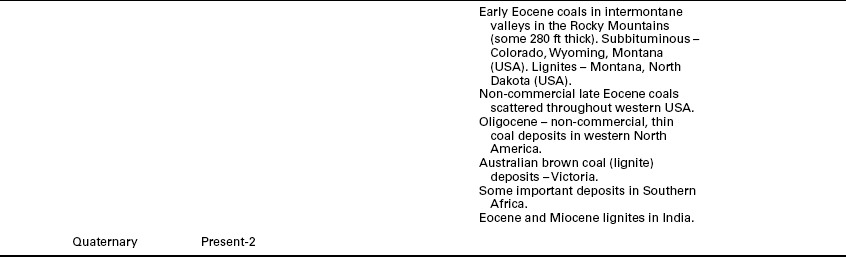
Source: Stach et al., 1975; Falcon, 1986; Falcon and Ham, 1988; University of California at Berkeley, 2011; University of Wyoming, 2002; Earth Science Australia, 2011; Geocraft, 2011; Scotese, 2011; Australian Mines Atlas, 2011; Khandelwal and Singh, 2010.
Climatic conditions during the major coal-forming periods varied greatly depending on the location of the land mass. For example, coals formed during the Pennsylvanian portion (320–286 mya) of the Carboniferous period in the United States and Canada were formed in a tropical climate with the equator running along a line stretching from central Colorado to Nova Scotia (Geocraft, 2011). The climate was relatively hot (global temperature of about 12 °C in the middle Carboniferous) and humid at that time (Geocraft, 2011), with expansive swamps containing plants that reached 45 m in height (Earth Science Australia, 2011).
The plant matter was buried at different depths. In the Appalachian coal fields of, for example, Pennsylvania, West Virginia, Ohio, Kentucky, and Virginia, the depth of burial was quite significant, though much of the sediment has eroded over time. With a geothermal gradient of 1 °F per 100 ft of depth (0.556 °C per 30.5 m), temperature with burial added greatly to the coalification history of these coals. These coals are of the high, medium, and low volatile bituminous coal ranks. Coals in the Interior Basin (Illinois and Indiana) were not buried to a similar extent. Some of these coals have achieved similar coal rank (high volatile bituminous) because of heating from igneous intrusions at depth (Damberger, 1974). This is significant, in that some of the Illinois and Indiana coals have bed moistures approaching or greater than 10% while similar rank eastern US coals have bed moistures of 1–2%. This is a result of the difference in the depth of burial, with less water being removed under pressure from the overlying sediment.
Conversely, coals in Australia, India, and South Africa were formed during a much cooler climate during the Permian period that followed the Carboniferous. During the Carboniferous, these areas formed the southern supercontinent of Gondwanaland and were covered by glaciers while the northern land mass (Laurasia) was near the equator, experiencing a significant coal-forming period. As the continents drifted north, Gondwanaland warmed and conditions developed that allowed many of the major coal deposits of the southern hemisphere to form (Falcon, 1986; Falcon and Ham, 1988; Stach et al., 1975).
After this major coal-forming period, the land masses collided to form one continent known as Pangea. It was during this time that the Appalachian Mountains in the eastern US formed, and the coals deposited in eastern Pennsylvania were subjected to this mountain-building. It is thought that this metamorphism of bright coal produced the extensive anthracite seams there (Roberts, 1924; White 1925).
As the earth’s plates continued to move, Pangea began to break apart. The climate became moist again and conditions, seemingly worldwide, were right for another period of coal formation. For example, many of the significant commercial western coals in the USA that are of the lignite and sub- bituminous rank were formed during the Cretaceous and Tertiary periods (University of Wyoming, 2002).
Some of the differences found in the major coal-forming periods are summarized in Table 2.2.
2.4.1 Peat swamp conditions and the presence of minerals
The original peat swamp conditions played a role in determining coal type and the association of minerals or inorganic compounds within the coal seam. Flooding could bring sediments from the surrounding land mass. The swamp could undergo periods of drainage when the organic matter would be exposed to oxygen and decompose more rapidly. In a coastal environment, the seas could bring an influx of salts. Differing conditions gave rise to different inorganic matter in coals, including the presence of, for example, salts, sulfates, sulfides, calcites, carbonates, silicates, and every possible trace element. Minerals in solution could be deposited within a coal (see Fig. 2.2) on a microscopic basis, limiting their removal today during coal preparation. Figure 2.2a shows pyrite formed inside a preserved plant cell in fusinite, while Fig. 2.2b shows finely dispersed pyrite in a coal sample. These were found in a clean coal product from a laboratory froth flotation cell.
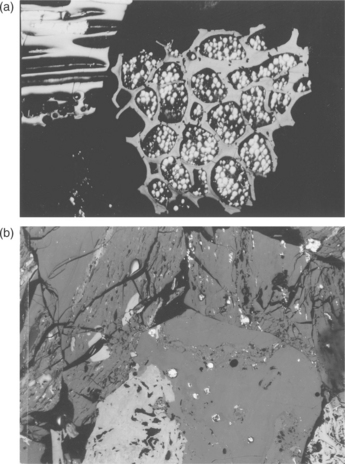
2.2 Pyrite filling a preserved plant cell in fusinite (a) and finely dispersed pyrite (b) in froth flotation clean coal products, showing the difficulty of separating some intimately associated minerals from Pittsburgh seam coal.
However, coal rank is not associated with the mineral content of the coal, but with the maturation or coalification of the organic matter. It is necessary therefore to consider coal rank based on a ‘pure coal’ basis. Most of the standard coal analyses are therefore compared on a ‘mineral matter free’ basis for coal rank determination.
This is done by calculating the mineral matter in coal, which is generally accepted to be calculated based on the Parr formula (Parr, 1928).
The mineral matter calculation is a combination of the analyses for ash and sulfur. Total ash is measured using standards developed for the buying and selling of coal and is determined under very specific conditions by heating a coal sample over time to a given temperature. During this analysis, the minerals that are present change – moisture from silicates and carbon dioxide from carbonates are lost. During the analysis for total sulfur content, sulfide minerals lose sulfur oxides. The Parr mineral matter calculation is an effort to reconstitute those minerals from the ash and sulfur analysis so that values for other analyses in rank determination can be adjusted to exclude the contribution of minerals.
Minerals in coal are often removed using coal preparation technologies. However, there are limits to mineral reduction based on the physical distribution of minerals within a coal seam. Some minerals form roof or floor rock or large partings and are liberated from the coal during mining. These are easily removed during coal preparation. However, other minerals are more intimately associated with coals and can be difficult to remove without also discarding coal. Other minerals are dispersed microscopically throughout a coal. Indeed, in some instances, finely dispersed pyritic sulfur has been mistaken for organically-bound sulfur using the standard analytical procedure.
Fortunately, most coal utilization technologies have been adapted to handle certain amounts and types of minerals. These are often given in coal contracts as limits to the total amount of ash or sulfur that is acceptable, especially for metallurgical uses. For metallurgical coals, importance is placed on the organic composition of the coal, as will be discussed in a later section.
Depending on the type and design of boiler in a coal-fired power plants, there are often limits placed on the types of minerals present, as well as the quantity, as these affect the slagging and fouling in a boiler. The analyses most often used in these determinations are the ash fusion temperature measurement and ash composition. Samples of ash are prepared from a coal and are analyzed according to the applicable standards given by the American Society of Testing and Materials (ASTM), the International Standards Organization (ISO), or other country-based standards organizations. A listing of standards is given in Table 2.3.
Table 2.3
Standards for various coal analyses
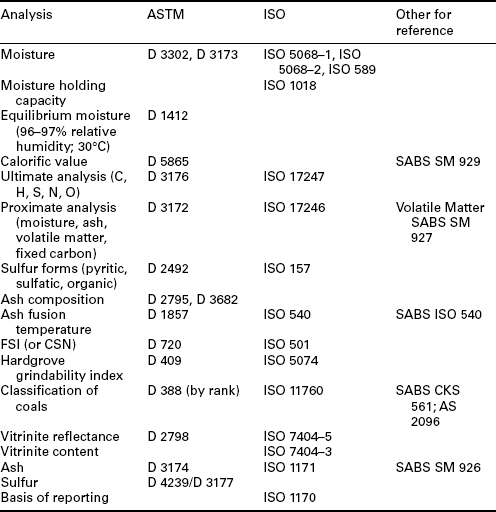
Source: South African Bureau of Standards, 2000; Sanders, 1974; ASTM, 2011; ISO, 2011.
The ash fusion temperature describes the melting behavior of a cone of ash – initial deformation, softening, hemispherical, and fluid. Temperatures are observed for each of these conditions. The test is conducted in both an oxidizing and a reducing atmosphere. High ash fusion temperatures are desirable in dry bottom boilers, while low ash fusion temperatures are desirable in a boiler handling molten ash.
For ash composition, also known as ash constituents or oxides of ash, the elements in ash are reported as the weight percent of a stable oxide – Si is reported as SiO2, Al as Al2O3, etc. The other oxides reported are TiO2, Fe2O3, CaO, MgO, Na2O, and K2O. The combination of these elements gives an indication of the slagging or fouling potential at a power plant. It will also influence the slag properties in a blast furnace, where alkalies should be limited.
2.5 Coal properties and standards for coal classification systems
Coal is bought and sold commercially based on standardized analyses. Every country uses its own methods and has its own standards, though through the ISO, worldwide coal analytical procedures are very similar. Specific standards for ASTM and ISO (or other standard organizations where available) are given in Table 2.3 for many important coal analyses.
2.5.1 Coal properties for utilization
In general, coal for thermal purposes is sold based on its as-received calorific value. In the US, the units are British thermal units per pound or Btu/ lb. Most other countries use MJ/kg as the units. The as-received calorific value is affected by moisture content and ash content. As moisture and ash increase, the calorific value decreases. To more precisely define the level of moisture or ash, coal analyses are reported on different bases as shown in Table 2.4.
Table 2.4

Example: A coal contains 13 000 Btu/lb on a dry basis. What is the as-received heating value at 6% as-received moisture?
Dry Btu/lb × (100 – As-received Moisture)/100 = As-received Btu/lb
13 000 × ((100 – 6)/100) = 13 000 × 0.94 = 12 502 Btu/lb (as-received)
The as-received analysis – that containing the moisture and ash content ‘as-received’ at a shipping point or destination – is typically used for thermal coal contracts. Analyses on a dry basis are often used for metallurgical coals, while a moisture limit is given in a contract.
Volatile matter content is also an important parameter for thermal coal – sufficient volatile matter must be present to sustain combustion in some boiler designs. Other boilers are specifically designed for low volatile anthracite. Some are designed for very high levels of ash (ash-forming minerals) and for different types of minerals, as discussed previously.
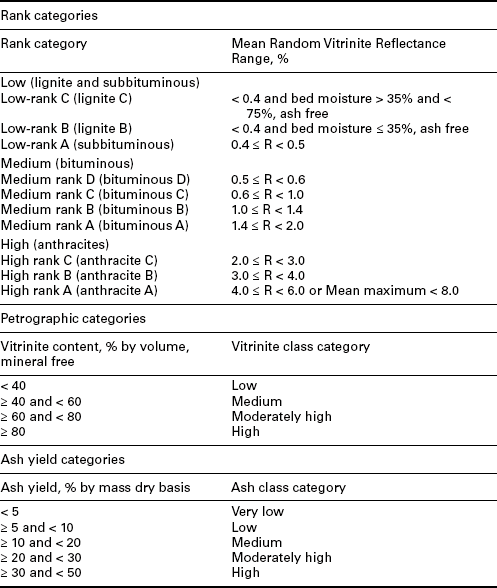
Several of these parameters are combined to give the ‘proximate’ analysis of coal – moisture, ash, volatile matter, and fixed carbon (determined by difference). These, along with heating value, are used in the ASTM classification for coals by rank (ASTM, 2005) as shown in Table 2.5. Heating value is used for the ‘low-rank’ coals as its value changes most consistently – an increase with increasing rank. Fixed carbon and volatile matter change consistently with the ‘high rank’ coals – an increase in fixed carbon and decrease in volatile matter with increasing rank. Note that as indicated previously, these values are given on a mineral matter free basis using the Parr formula (Equation [2.1]). For fixed carbon and volatile matter, these are on a dry, mineral matter free basis, while for heating value, the bed or equilibrium moisture is included – a moist, mineral matter free basis. The ASTM standard (ASTM, 2005) includes other details for these calculations including adjustments for SO3 in the ash. Several examples of the determination of coal rank are given for coal samples in the standard following all of the guidelines specified. The most important feature is that these values are then on a ‘pure coal’ basis for the determination of coalification or rank.
Table 2.5
ASTM classification of coals by rank
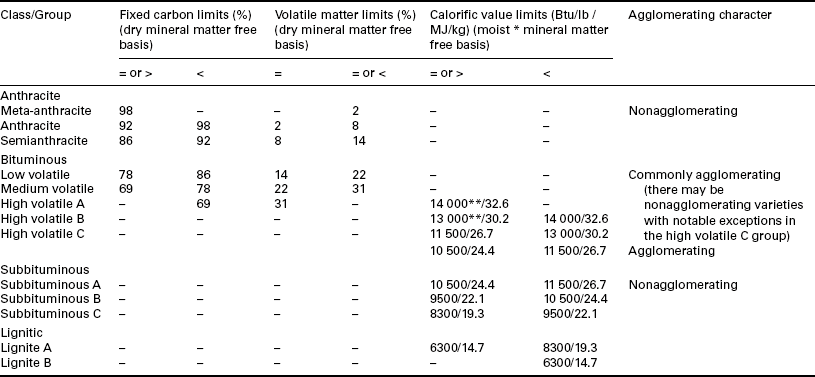
This classification does not include a few coals, principally nonbanded varieties, which have unusual physical and chemical properties and which come within the limits of fixed carbon or calorific value of the high volatile bituminous and subbituminous ranks. All these coals either contain less than 48% dry mineral matter free fixed carbon or have more than 15 500 moist mineral matter free Btu per pound (Btu per pound 2326 = kilojoules per kilogram).
⁎Moist refers to coal containing its natural inherent moisture but not including visible water on the surface of the coal.
⁎⁎Coals having 69% or more fixed carbon on the dry mineral matter free basis shall be classified according to the fixed carbon, regardless of calorific value.
Source: ASTM, 2005.
Total moisture is analyzed as part of the proximate analysis. However, as mentioned previously, another type of moisture – bed or inherent moisture (also sometimes called equilibrium moisture) – is used in the ASTM rank classification. Total moisture is a combination of inherent moisture and surface moisture – free moisture on the surface of coal. Surface moisture can change rapidly through air drying or exposure to moisture. Inherent moisture decreases with increasing coal rank and is measured after holding a coal sample at a specified temperature and relative humidity for a given time. Lignites can contain 30% or more inherent moisture, subbituminous coals about 25%, lower rank bituminous coals about 10% inherent moisture, and high rank bituminous coals about 1–2% inherent moisture. Moisture content is always part of a coal contract, as it will affect boiler performance and, in the case of surface moisture, the handling characteristics of the coal – too much surface moisture can lead to difficulties in flow through chutes and bins (Arnold, 1995).
The ‘ultimate’ analysis of coal includes elements that are present in coal – carbon, hydrogen, sulfur, nitrogen, and oxygen (determined by difference). Carbon, most notably, increases with increasing coal rank, while oxygen decreases (see especially Table 2.1). The oxidation of a coal is often an important parameter for coal utilization technologies, especially if a coal is extremely weathered or high in oxygen. It may indicate a high fusinite content, which is an unreactive maceral – very high levels may be undesirable for some metallurgical applications, though some is desirable as it can add to coke strength. And, fusinite may be recovered to a lesser degree in the coal cleaning process known as froth flotation, which is based on the hydrophobicity of the coal surface (Arnold and Aplan, 1989).
As mentioned earlier, the properties of the organic matter in coal are of special importance for metallurgical uses. Limits are placed on ash and sulfur, but other laboratory analyses are used to determine ‘coking’ properties. These tests provide the crucible swelling number (CSN) or free swelling index (FSI). The mean maximum vitrinite reflectance, which changes most consistently with coal rank, is also important for metallurgical uses. In addition to the vitrinite, other macerals are expected in the metallurgical coal product to optimize the coal for the final use. For example, inertinite macerals present in the coal will add to the coke strength. Later chapters on utilization will refer to these parameters.
One other important property that changes with coal is the grindability of the coal. The Hardgrove grindability index is a measure of this property that indicates the ease of grinding the coal and gives a measure of its friability. Lignites have low HGI values and are difficult to grind. A high volatile A bituminous coal may have an HGI of about 55, while a low volatile bituminous coal may have an HGI of over 100. HGI reaches a maximum at this rank and is lower for the harder anthracites. For high HGI coals, extra care is often taken in coal handling and preparation to reduce the amount of fines generated. More energy is required to mine coals that have a low HGI. As coals are crushed to approximately 6 mm topsize for cyclone boilers and pulverized to 0.075 mm for pulverized coal boilers, HGI will also have an impact on the milling requirements in a power plant. The coals with the maximum HGI (easiest to grind, most friable) are generally at the peak for coking coals, as this is also where vitrinite reflectance and other parameters peak.
2.5.2 Coal classification systems
Coal classification systems have been developed by many investigators on the basis of various coal analyses. One of the earliest was that of Seyler (Sanders, 1994) that categorized coal based on carbon and hydrogen content on a ‘pure coal’ or dry mineral matter free basis, which adjusted the carbon and hydrogen based on the Parr mineral matter formula, as discussed previously. It was modified later to include calorific value and volatile matter, oxygen, air-dry moisture contents, and CSN to extend it to lignites (brown coals). It was found that, though this was developed for British Carboniferous coals, it also worked well for Australian Permian coals (Sanders, 1994).
As presented previously (Table 2.5), the ASTM coal rank classification is based on chemical parameters for coal that change with rank (heating value, fixed carbon or volatile matter). Going beyond coal rank classification, Australian Standard AS 2096 (Sanders, 1994) classifies coals using a numeric code (REVMAS code for lower rank and REVCAS code for higher rank) for a given coal. It is based on mean maximum reflectance of vitrinite (R), gross specific energy – dry ash free (E), volatile matter – dry ash free (V), bed moisture for lower rank coals (M) or CSN for higher rank coals (C), ash percentage – dry (A), and total sulfur – dry (S).
ISO prepared a coal classification standard in 2005 (ISO, 2005) based on vitrinite reflectance (mean random) for coal rank. In this overall standard, ISO included classifications for petrographic composition (amount of vitrinite) and ash classification. The ISO coal classification standard (ISO, 2005) is summarized in Table 2.6. The highest ash category for ‘coal’ is given as ≥ 30% and < 50% ash. Above this, presumably, a sample is no longer designated ‘coal.’ However, there are some resources discarded from previous mining and coal preparation activities that are higher than 50% ash content and that are being pursued for rewashing, including for the production of some metallurgical coal products.
As coal is traded globally, reference to standards is becoming increasingly important for understanding the character of the coal for a given application. Reporting coal on a particular basis is essential in buying and selling coal worldwide.
2.6 Sources of further information and advice
The internet has become a significant source of information on a wide variety of topics, and information on coal is prevalent. However, it is best to ensure that the source is reliable. Many universities and coal associations produce reliable information. The standards for coal analytical as well as some engineering procedures from ISO and ASTM are found at websites for purchase (ASTM, 2011; ISO, 2011).
However, there are still classic references for coal properties and chemistry, coal rank, and coal petrography/petrology. Stach’s textbook of coal petrology (Stach et al., 1975) and Coal (van Krevelen, 1961) are still important resources for coal investigators because of their thorough treatment of the subject matter.
2.7 Future trends
As coal continues to be sold internationally into both thermal and metallurgical markets, coal analytical procedures will be honed. It will not be possible to overlook the role of macerals in the combustion of coal. Coal mining and preparation engineers will need to understand how the differences in macroscopic and microscopic coal types affect the mining and processing of coals. A recent meeting attended by the author in British Columbia, Canada, raised the suspicion that coal was not as easily recovered in froth flotation as it should have been because of the presence of fusinite, though other rank parameters would have indicated that the coal should be recovered quite easily (Arnold and Aplan, 1989). Fusinite content will also affect the utilization of the coal and determine if it is best used by the thermal, PCI, or traditional coking coal market. The role of macerals in the mine and through the final coal product must be understood.
This chapter does not cover the coal analyses required for effective coal preparation plant design and performance testing. There will be a need to revisit much of the work conducted in the past on the separation of coal macerals in order to optimize coal preparation processes to produce coal products that are tailored for a specific end use, as the use of coal for producing liquids, gasses or other high value carbon products expands.
2.8 References
American Society for Testing, Materials, Standard classification of coals by rank, 2005:D 388.
American Society for Testing and Materials. Standards. Available from: http://www.astm.org/Standard/index.shtml, 2011. [[Accessed 28 September 2011]].
Arnold, B.J. A guide to coal handling. Palo Alto: Electric Power Research Institute; 1995. [TR-105110.].
Arnold, B.J., Aplan, F.F. The hydrophobicity of coal macerals. Fuel. 1989; 68:651–658.
Australian Mines Atlas. Coal fact sheet. Available from: http://australianmine-satlas.gov.au/educatoin/fact_sheets/coaljsp, 2011. [[Accessed 28 September 2011].].
Damberger, H.H., Coalification patterns of Pennsylvanian coal basins of the eastern United States. Spec. Pap. 153. Geol. Soc, Amer, 1974:53–74.
Earth Science Australia. Geological time periods. Available at: http://earthsci.org/fossils/youngp/periods/periods.html, 2011. [[Accessed 28 September 2011].].
Falcon, R., A brief review of the origin, formation and distribution of coal in Southern AfricaAnhaeusser, C.R., Maske, S., eds. Mineral deposits of Southern Africa. Geol. Soc. S. Africa, Johannesburg, 1986:1879–1898. [1 & 2].
Falcon, R., Ham, A.J. The characteristics of Southern African coals. J. S. Afr. Inst. Min. Metall.. 1988; 88(5):145–161.
Geocraft. Plant fossils of West Virginia. Available from: http://www.geocraft.com/WVFossils/Carboniferous_climate.html, 2011. [[Accessed 28 July 2011].].
Given, P.H., Short course on coal characteristics and coal conversion processes. The Pennsylvania State University, State College, 1975:19–23. [May.].
International Standards Organization, Classification of coals, 2005. [ISO 11760:2005(E).].
International Standards Organization. ISO Standards. Available from: http://www.iso.org/iso/iso_catalogue.htm, 2011. [[Accessed 28 September 2011]].
Khandelwal, M., Singh, T.N. Prediction of macerals content of Indian coals from proximate and ultimate analyses using artificial neural networks. Fuel. 2010; 89:1101–1109.
McClung, J.D., Geer, M.R., Properties of coal and coal impuritiesLeonard, J.W., eds. Coal Preparation. AIME, New York, 1979. [1-1–1-79.].
Parr, S.W., The classification of coal. Bulletin No. 180. Engineering Experiment Station, University of Illinois, 1928.
Radovic, L.R., Schobert, H.H. Energy and Fuels in Society. New York: McGraw-Hill, Inc.; 1992.
Roberts, J. The origins of anthracite. Proceedings. 1924; 40:97–138. [SW Inst. Eng].
Sanders, R.H., Coal characterizationSwanson, A.R., Partridge, A.C., eds. Advanced coal preparation monograph series. Australian Coal Preparation Society, 1994. [1 (1)].
Scotese, C.R. Paleomap project. Available from: http://www.scotese.com/moreinfo7.htm, 2011. [[Accessed 28 July 2011]].
Stach, E., Mackowsky, M.-T.H., Teichmuller, M., Taylor, G.H., Chandra, D., Teichmuller, R. Stach’s Textbook of Coal Petrology. Berlin: Gebrüder Borntraeger; 1975.
South African Bureau of Standards, Anthracitic and bituminous coals, 2000. [CKS 561.].
University of California at Berkeley. The Carboniferous. Available from: http://www.ucmp.berkely.edu/carboniferous/carboniferous/html, 2011. [[Accessed 28 September 2011].].
University of Wyoming SMTC. Coal over geologic time. Available from: http://smtc.uwyo.edu, 2002. [[Accessed 28 July 2011].].
Van Krevelen, D.W. Coal. Amsterdam: Elsevier; 1961.
White, D. Progressive regional carbonization of coals. Transactions, AIME. 1925; 71:253–281.