Coal petrography
Abstract:
This chapter describes the basics of petrographic analysis including lithology, the classification of macerals, minerals and their associated rocks, the concept of rank, and the impact that these factors have on the technological properties of coal. The development of modern techniques towards grain analysis and mineral and maceral transitions is briefly described.
3.1 Introduction
Coal is defined in the Oxford Dictionary as ‘a combustible black or dark brown rock consisting chiefly of carbonised plant matter, found mainly in underground seams and used as fuel’. The word ‘coal’ is derived from Old English col (in the senses of ‘glowing ember’ and ‘charred remnant’) and is related to Dutch kool and German Kohle. More conventionally, coal is defined as a readily combustible sedimentary rock composed of organic materials derived from ancient plants with an ash content less than 50% by mass on a dry basis. The question arises, however, of what to call black combustible carbonaceous ‘rocks’ with ash content greater than 50% that are currently being used successfully for power generation in some countries of the world. It may be argued that the definition of coal should therefore include such rocks with higher ash content. But whether they have higher ash content or not, not all such coals or ‘black or dark brown’ rocks burn successfully. The answer lies in the organic and inorganic matter content contained within those ‘rocks’. It is the origin, formation and nature of those components that will be discussed in this chapter, with comments on their technological properties and performance in some utilization technologies.
The origin of coal lies in a set of circumstances that prevailed at the time of original peat swamp formation and subsequently during the process of coalification (maturing) through time, temperature and pressure. The lithology of coal as defined by the American Society for Testing and Materials (ASTM) is ‘the term used to describe the coal bed [or seam] and its structural aspects which are obtained from macroscopic and microscopic observations of coal’.
As discussed in Chapter 2, coal-forming swamps that resulted in the coal seams being mined today formed in a variety of different environments and it is these that controlled the nature and properties of the organic and inorganic composition in coal. The environments range from upper and lower deltas on the shorelines of marine seas or inland fresh water basins, in wide lowland coastal swamps, in lakes and valleys in inland mountainous regions and along river-cut mature plains. The environments in such diverse locations varied from water-logged to intermittently wet and drier environments, while geochemical environments varied, subject to the nature of the swamp waters. These ranged from fresh waters supplied by melting glaciers or rain-sourced rivers from hinterland sources to brackish and marine-sourced waters on the shores of ancient seas.
Such variety gives rise to a multitude of geochemical conditions in peat swamps, ranging from acidic to alkaline, and anaerobic (reducing) to aerobic (oxidising) environments. Equivalents today may be seen in the Okefenokee Swamps of Florida, the mangrove swamps of Malaysia, the deltas of the Mississippi and Amazon rivers, the cool temperate rain forests along the southern shoreline of Alaska, and the peat bogs of Ireland which formed on the low-lying, water-logged uneven ground left after the last Ice Age.
Amongst the best known coals being mined commercially today are those that formed during the Carboniferous of the northern hemisphere in the supercontinent of Laurasia and during the Permian and Triassic periods of the southern hemisphere in the supercontinent of Gondwana. In the Laurasian coals swamps, tall fleshy lycopods, ground pines and club mosses flourished in extensive water-logged swamps in hot humid equatorial climates. In the Gondwana swamps, forests of tall and shrub-like woody Glossopteris-leafed trees grew in cold to cool temperate conditions with alternating short hot summers and long cold winters as the climate warmed up after the long period of Dwyka sheet glaciation which had covered much of that supercontinent during the Carboniferous.
The vegetation, climates and environments of accumulation were therefore significantly different in the peat-forming swamps of the supercontinents of Laurasia and Gondwanaland, both of which have given rise to what is known as mature or hard coals, namely bituminous and anthracite deposits.
More recent coal-forming times also occurred in both the Northern and Southern hemispheres during the Cretaceous and tertiary periods. These coals, however, have not matured to the same degree as the Laurasian and Gondwana hard coals. Known as lignites (or brown coals) and subbituminous coals, they are different in composition, texture and physical and chemical properties (see Chapter 2). The terminology and descriptions of those coals are not included in this chapter.
In each geographical region during Carboniferous, Permian and Triassic times, the vegetation provided further unique ranges of plant organs (roots, stems, branches, leaves, fruits, flowers, pollens and spores) and plant tissues, all of which possessed different physical structures and chemical composition. These decomposed to varying degrees during the process of peatification, i.e. the biochemical conversion of plant material by anaerobic or aerobic bacterial action which occurs in different ways subject to the sedimentary, climatic and geochemical conditions at the time of peat accumulation. It is the fossilised remains of these original plants that comprise the final organic fragments found in coal. Known as macerals, they are defined as the small, microscopically identifiable units of altered organic plant remains seen under the petrographic microscope. Collectively, they give rise to the type of coal seen macroscopically (observable to the naked eye), namely the bright, dull and banded layers or lithotypes in coal seams. Associated with the organic matter (macerals) is a variety of minerals of different types, in various forms and in different degrees of association. The association of macerals and minerals in microscopic bands (> 50 μm) within a coal seam comprise the microscopically defined bands called microlithotypes found in coal.
Maceral names have the ‘-inite’ suffix (e.g. vitrinite), whereas microlithotypes have the ‘-ite’ suffix (e.g. vitrite). Lithotypes have the ‘-ain’ suffix (e.g. vitrain). Coal petrography is the technique used to identify, examine, classify and apply the information obtained from a microscopic study of these entities in coal. These will be discussed further below.
3.2 Macerals and minerals in coal
This section introduces, defines and describes the microscopic organic and inorganic components in coal.
3.2.1 Macerals
The organic fragments in coal have been classified into three groups based on major characteristics pertaining to each group. These are the vitrinite maceral group, the inertinite maceral group and the liptinite maceral group. Each is subdivided into further macerals and sub-macerals.
Woody tissue (bark, cortex and xylem) falling into anaerobic water-logged conditions, such as prevailed over much of the hot equatorial swamps of Laurasia, over time becomes gelified, thereby trapping in the protoplasmic contents of the cells and softening the tough lignitic or cellulosic cell walls. Humic gels and humic acids are leached out and precipitated in layers alongside the gelifying woody materials (logs, branches, twigs and roots) or in the empty cells of woody tissues. In time, with the pressure of burden due to burial by overlying sediments or depth through mountain-building movements with faulting or graben structures, the gelified matter becomes compressed. In so doing, it loses moisture and volatiles, and ultimately hardens into the bright shiny bands seen in the bituminous and anthracitic coals of today.
The process of gelification or vitrinitisation (derived from an original French word meaning ‘to gelify’) gave rise to the term vitrinite in bituminous or anthracitic coals, i.e. in the hard coal range of rank. In lignites, between peat and the hard coal range, similar gelified materials are included in the huminite group of macerals.
The vitrinite maceral group is grey in colour with a reflectance generally between that of associated darker liptinites and lighter inertinites over the range of rank in which the three respective macerals can be readily recognised. The group includes three subgroups and six macerals derived from different humic materials and their varying pathways of transformation within the early peats – see Table 3.1. The three main subgroups include (i) relatively pure layers or lenses of homogeneous gelified material (Fig. 3.1a, telocollinite), some of which may also be found as amorphous cell fillings or as groundmass binding other coal components (Fig. 3.2, desmocollinite); (ii) well-preserved cell walls (Fig. 3.1b, telinite); and (iii) detrital fragments in an association of detrital organic and mineral materials (vitrodetrinite).
Table 3.1
Macerals within the vitrinite group in bituminous and anthracite coals
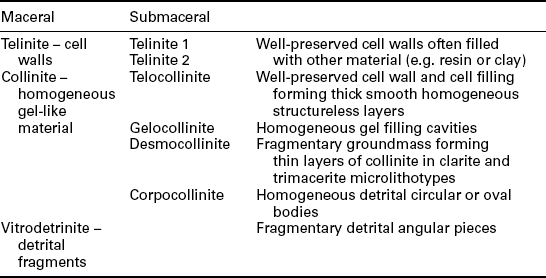
Lignite macerals have different nomenclature. These have not been included here.
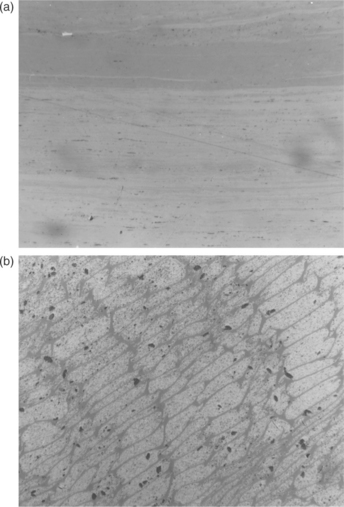
3.1 Vitrinite (a) Telocollinite (thick smooth structureless layers of vitrinite. (b) Telinite (vitrinite seen as well-preserved grey cell walls, cells filled with granular white micrinite). Scale 200 μm from side to side.
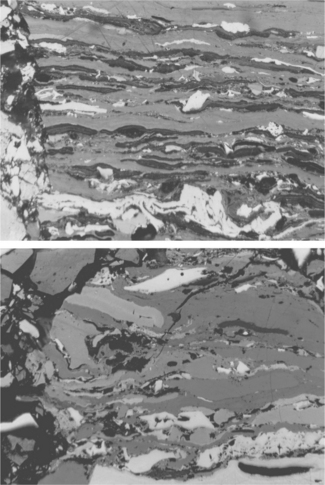
3.2 Vitrinite – desmocollinite (grey layers comprising the matrix in which spores and pollen (liptinite-sporinite, dark grey thin, black, narrow and lense-like) and fragments of inertinite (white) are embedded). Scale 200 μm left to right.
The chemical structure of vitrinite, in simple terms, is moderately aromatic with significant aliphatic chains in the bituminous range of rank. In comparison, inertinite is more structurally ordered and aromatic while liptinite is predominantly aliphatic. On heating vitrinite, volatile matter derived from the breakdown of the aliphatic chains is released relatively easily, and the remaining aromatic carbon-rich molecular structure softens, swells and becomes porous to varying degrees, subject to the level of rank of the coal.
Plant material which accumulates in drier environments with exposure to varying levels of aerobic conditions produces a variety of different organic fragments. Under these conditions, desiccation (drying out) and oxidation takes place, plant tissues are attacked by microbial fungi and bacteria to varying degrees, and some plant material may have been subjected to fire. If taken to extremes, such conditions lead to the complete destruction of content within the cell tissues, thereby leaving the most resistant, tough, fibrous outer cell wall material, namely lignin and cellulose, for burial in the accumulating peat swamp. In general, this maceral group is dense and structurally disordered, with tightly bonded aromatic carbon molecules and few if any aliphatic chains. As a consequence, this group produces limited or no volatile matter on heating, thereby rendering it relatively difficult to ignite and to burn out due to its dense molecular structure. For these reasons, this maceral group was considered to be inert during combustion and, as such, was termed ‘inertinite’. Typically the colour is white with a reflectance lighter than either vitrinite or liptinite in low to medium rank coals.
A wide range of forms are known in the inertinite maceral group. These range from fossil wood that had been burnt in ancient fires, resulting in dry, brittle, lightweight particles of porous strongly cellular fossil charcoal called ‘fusinite’. Under the petrographic microscope this maceral has a well-preserved cellular structure and appears hard (high relief), highly reflective, and very bright white to brassy yellow in colour. It occurs in stratified layers in a seam (possibly the remains of burnt logs, tree trunks and branches) or as broken fragments dispersed within the coally matrix – see Figs 3.3 and 3.4.
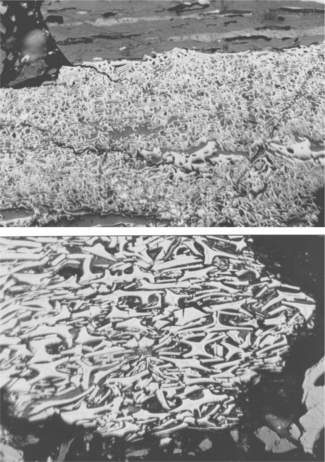
3.3 Inertinite – fusinite (highly reflecting, strongly structured, white compressed cell walls of ancient woody material). Scale 200 μm from side to side.
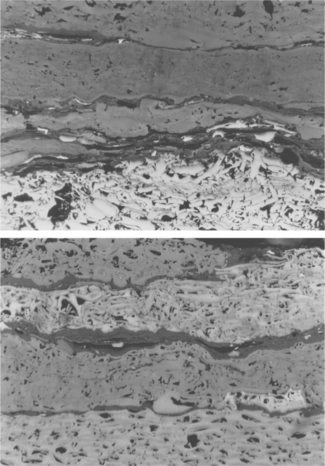
3.4 Inertinite – semifusinite (inert (pale grey) and semi-reactive (darker grey) layers of partially structured and semi-gelified woody tissues) and fusinite (strongly structured, highly reflecting, white layers of woody tissue). Scale 200 μm side to side.
Fibrous organic fragments which display clear fibrous woody cell structure but lower levels of reflectance than fusinite with colours varying from white to very pale grey and varying degrees of gelification are termed ‘semifusinite’. Those semifusinites closer in structure, colour and reflectance to fusinite have been termed ‘inert semifusinite’, whereas those closer to vitrinite in terms of semi-gelified structure, greyer colour than fusinite but higher reflectance than vitrinite (indicating higher volatile matter content in this inertinite group) are termed ‘reactive semifusinite’. Such materials are particularly prevalent in Gondwana coals, but are relatively rare in the Laurasian coals – see Fig. 3.4.
Inertinite group macerals that have been broken into fragments between 2 and 30 μm in size are termed inertodetrinite – see Fig. 3.5. Subject to their colour and reflectance, these detrital fragments may be classified as inert or reactive in technological processes, in a manner similar to that applied to semifusinites. Such inertinite fragments are typically abundant in many Gondwana coal seams, often comprising the predominant maceral in thick seams or thick bands within seams, but they are not common in the Laurasian coalfields. These components are frequently found intimately associated with fine clay or quartz grains and, as such, are believed to have been removed from their original peat swamps and redeposited with silt and sand elsewhere as a result of the turbulent movement of water. Known as ‘drift coals’ they may have arisen, for example, through flooding by rivers bursting their banks following the seasonal melting of snow in the mountains surrounding the Gondwana coal-forming basins, the latter occurring after the cessation of the Carboniferous Ice Age in the southern hemisphere.
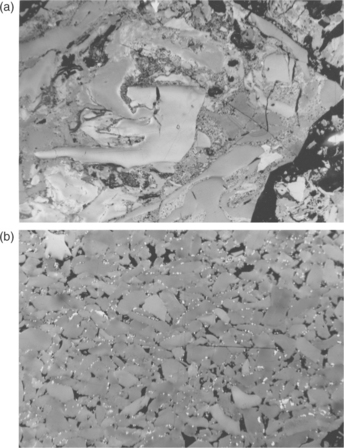
3.5 Inertinite – inertodetrinite (a) Large irregularly-shaped, unevenly- sized fragments of ancient plants cemented together with a micrinite (fine granular) matrix. (b) Small, rounded, evenly-sized fragments compacted into a layer with very fine clay (black) and pyrite grains (bright, white, minute crystals). Scale 200 μm from side to side.
Also included in the inertinite group of macerals are secretinite (sclero- tinite), macrinite and micrinite. These components share the same white to pale grey colour of the inertinite group but vary in origin, structure and texture. Micrinite is composed of very small granules of inertinite (< 2 μm or less) found in concentrated lenses in vitrinite or inertinite, as fillings in the cellular cavities of fibrous woody tissue, or as the matrix in a collection of fragmented particles. This maceral is considered to be the solid residue of former oily, waxy or bitumen-like material that originated from liptinitic or hydrogen-rich vitrinitic substances. Macrinite occurs as an amorphous groundmass or as rounded fragments of discrete structureless bodies greater than 30 μm in size. It is analogous to collinite of the vitrinite group and is considered to have originated from flocculated humic substances which underwent dehydration and partial oxidation processes in an early stage during peatification due to temporarily lowered groundwater levels. Secretinite (previously known as sclerotinite) is composed of round or oval discrete bodies of fungal or cellular origin with well-preserved botanical structures in the centre (pores or shrinkage cracks). A summary of the characteristic forms in the inertinite group of macerals is presented in Table 3.2.
Table 3.2
Macerals within the inertinite group
| Maceral | Submaceral | |
| Fusinite | Pyrofusinite | Well-preserved cell walls (extreme oxidation) |
| Degrado-fusinite | Fragmentary detrital pieces of pyrofusinite | |
| Semifusinite | Well to semi-preserved cell walls (partial oxidation) | |
| Secretinite (Sclerotinite) | Rounded to oval bodies of fungal or cellular origin with well-preserved botanical structures (pores and shrinkage cracks) in the centre | |
| Micrinite | Small granules less than 2 μm in size | |
| Macrinite | Amorphous groundmass structureless rounded fragments larger than 30 μm in size (analogous to the collinite of the vitrinite group) | |
| Inertodetrinite | Detrital fragments of the above forms without any woody cell structure; 2–30 μm in size |
Apart from the fibrous, woody and fleshy tissues of trees and plants which gave rise to the macerals and maceral groups described above, another part of ancient plants needs to be considered. Known as the Liptinite group of macerals (previously the exinite group), these components originate from specialised hydrogen, waxy and oil-rich plant materials. Cutinite represents the waxy cuticles coating leaves and young shoots while sporinite represents the external coats (exines) of pollens and spores. This group also includes resin (Resinite) that exuded from the from the bark of ancient gymnospermous-like trees and algal bodies (Alginite), which occur either in concentrated layers or as discrete bodies within the coally matrix or in associated shales.
Liptinite macerals have characteristically low reflectances. They are brown to purple in colour in sub-bituminous and low rank bituminous coals (bituminous C and D) but range from grey to pale grey in the mid bituminous rank, after which they merge with vitrinite and disappear. The group is predominantly aliphatic in molecular structure and low in density. On heating, liptinite components in the bituminous C and D ranks of coal emit high proportions of H-enriched volatile matter followed by light and heavy hydrocarbons (bitumen, tars and waxes). Once emitted, little or no solid aromatic carbon remains. The macerals in the liptinite group are listed in Table 3.3.
Table 3.3
Macerals within the liptinite (exinite) group
| Maceral | Maceral variety | |
| Sporinite | Tenuisporinite Crassisporinite Microsporinite Macrosporinite |
Micro- macro- and megaspore exines (outer protective coats); usually compressed, well-preserved, distinct botanical forms |
| Cutinite | Elongated, usually saw-toothed, well- preserved; distinct botanical forms | |
| Alginite | Algal colonies with rounded, elongate, semi-compressed; distinct well- preserved in botanical forms | |
| Resinite | Globular or irregular bodies; discrete forms, filling cellular cavities or amorphous shapes in vitrinitic lenses | |
| Liptodetrinite | Small detrital fragments of the above forms |
3.2.2 Minerals
Minerals occur in various forms in coal. Minerals that were deposited at the same time as peat formation are termed syngenetic minerals, whereas those that formed after peat formation and at any time during coalification are epigenetic minerals, The latter minerals are usually derived from groundwater passing through cracks and fissures in the hardening or hardened coal seams. When saturated, the ions and elements in the ground water precipitate out and minerals form, taking the shape of the cracks or fissures in which they are deposited. Similarly, water passing through the xylem, phloem or cortical cells (supportive and ‘feeding’ cellular tissue) in ancient tree trunks also led to the precipitation of minerals in those cellular cavities, thereby ‘locking’ the minerals into those cell structures.
For practical purposes minerals may also be described as inherent, included or excluded. Inherent refers to minerals or elements bound to the organic cellular structure in coal (this includes trace elements which may have been taken up by the plant during growth and adsorbed into the plant’s tissues). Included mineral matter is that which forms a discrete mineral grain within the coally matrix, for example mineral grains of quartz and clay embedded in bands of vitrinite or inertinite in the manner of currants within a bun. Excluded mineral matter is that which is external to the coal or coal seam, namely particles of sandstone or shale which may have been derived from roof or floor rocks above or below the coal seam or from rock partings within the coal seam.
Within coal, the form in which mineral matter is found varies from extensive mineralised layers precipitated within the organic matrix to nodules, smaller grains, tiny crystals, fissure fillings, cell cavity fillings, or rock fragments which may be finely distributed throughout the coally matrix.
Within the syngenetic mineral category, those forms washed by water or wind blown into the peat swamp are predominantly clays and quartz and to a lesser extent feldspar, apatite, muscovite and related minerals. Those forms that precipitated in situ from colloidal, chemical and biochemical processes include pyrite, carbonate minerals and colloidal clays. Both groups are found intimately embedded within the organic matrix and are generally difficult to liberate during beneficiation. The epigenetic minerals that were precipitated during the later stages of coalification are generally found either in cleats and fissures or intruding into and disrupting the original layers of organic material. These include carbonate minerals (calcite, dolomite, ankerite, siderite), pyrite, marcasite and some clays. These may be coarsely crystalline or in the shape of nodules; in some cases minerals have been found to replace, transform or encapsulate older minerals. Carbonates that precipitate out in the cellular cavities of old tree trunks lying horizontally in peat have been found to be the hardest products known in coal. Called ‘hard fusite’ and comprising the fibrous cell walls (fusite) with calcite- or dolomite-filled cell cavities, such coally lithotypes have been found to wear mining picks away at extremely fast rates and be totally resistant to breakage in mills.
The most common minerals found in coal include the following:
Clay is overall the most abundant and widely spread mineral in coal. In the Gondwana coals, it often comprises up to 80% of the minerals present. It occurs as abundant small grains (from 1 to 2 μm up to 20 μm), is widely and finely distributed in the coally matrix, and is occasionally found as narrow lenses within the organic matrix or as thicker layers containing organic fragments. Some clay forms found filling cell cavities are considered to have formed by flocculation in situ and in many cases these fluoresce under ultraviolet light. Kaolinite is the most common of the clay minerals, although illite is also known to occur in many coalfields. When seen under the petrographic microscope, clays appear orange–brown to brown and black with an uneven granular structure.
Quartz is common in some seams, ranging in size from 5 to 50 μm. Often difficult to identify in oil immersion under the petrographic microscope, quartz grains have the appearance of glass. For these reasons, they are best viewed using other techniques (CCSEM and QEMSCAN). The form and shape of the grains range from rounded to angular, subject to their provenance and sedimentary history. Some grains bear rods of rutile within them.
Pyrite and marcasite, the most common iron sulphide minerals, are found to varying extents in all coals. Gondwana coals are generally low in iron sulphide minerals with minor exceptions, whereas these minerals are more common in Laurasian coals. Seen under the petrographic microscope they are bright yellow (up to 50 times brighter than the surrounding coal) and hard, generally exhibiting high relief. Pyritic minerals range from minute grains (1–2 μm) to large nodules (1 cm or more), as clusters of grains (framboids), and as lenses spread along strata within a seam. They are also frequently found as epigenetic minerals in cleats and fissures and as infillings in the cell cavities of massive woody fibrous bands of fusinite or semifusinites – see Fig. 3.6.
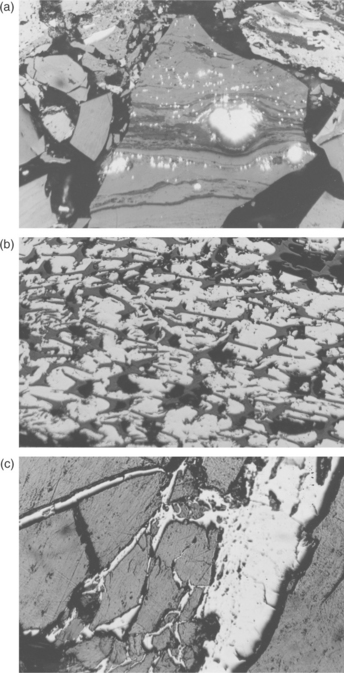
3.6 Minerals – pyrite – Various forms (a) very small to larger nodules in a vitrinitic matrix, (b) infilling cells in a fusinite layer and (c) precipitates in cracks and fissures intruding into a vitrinite layer. Scale 200 μm from side to side.
Carbonate minerals (calcite, dolomite, ankerite and siderite) are found both as syngenetic nodules within the organic matrix and in epigenetic forms as lenses intruded into early peats, as cell fillings, or as cleat deposits.
Other less frequent minerals include, but are not exclusive to, the following: feldspar, apatite, barite, gypsum, sphalerite and zircon. Radioactive forms of the latter mineral have been found in southern Africa.
Minerals in coal are important in the processes to which coal is exposed. Quartz is highly abrasive and is responsible for rapid wear in mills and ducts; clays are difficult to remove in beneficiation because of their very fine size, intimate intergrowth within the coally matrix, and often abundant distribution. Pyrite in cleat form is easier to remove due to ease of liberation during crushing relative to the forms of fine crystals embedded within organic matrices. Iron sulphide minerals emit SO2, a greenhouse gas, on heating. Carbonates are brittle, emit CO2 at low temperatures on heating, and play a role in reducing ash fusion temperatures when included in coal destined for a boiler. Collectively, minerals reduce heat when used for power generation and, subject to conditions in the boiler, certain forms can lead to slagging and fouling. They also reduce coking capacity if present in quantities higher than 14% ad.
3.3 Lithotypes, microlithotypes and abnormal conditions found in coal
This section outlines the macroscopically visible bands or lithotypes in coal, the conditions under which they formed, the variations in quality that arise laterally and vertically and the abnormal conditions found in coal.
3.3.1 Lithotypes and microlithotypes
In some cases, ancient peat-forming plant assemblages grew and died in situ, accumulating vegetation in the same swamp over time, fluctuating only in water level and geochemical environment over that time. Known as ‘autochthonous’ or in situ coals, these exhibit banded or stratified layers of vitrinite-rich, inertinite-rich or mixed organic matter contents. In hand specimen, these coals exhibit either bright (vitrinite-rich or vitrain) or dull (inertinite-rich or durain) bands or, in the case of multiple alternating narrow lenses of vitrinite and inertinite-rich layers, mixed banded coals. Known as lithotypes, the description ‘bright, dull or banded’ is a reflection of smaller microscopic bands of associated macerals and minerals. The latter are known as microlithotypes, and are defined in 50 μm bands of thickness.
Under conditions where developing peat swamps have been disrupted and broken up, either by flooding rivers, melting snows or incursions of the sea, the fragmented organic materials and accompanying minerals are carried in suspension to different locations and redeposited in the process of sedimentation in a manner similar to a river carrying sediments of sand and silt, which are deposited when the river meets a larger body of water. Suspended materials sink to the bottom, heaviest first, lightest last. In this manner, larger organic fragments and mineral grains are deposited before finer fragments in such a body of water. Coals formed in this manner are known as ‘allochthanous’ or drift coals, i.e. the fragmented materials have been removed, transported and deposited in a location different from their origin and have accumulated there to form the seam that is seen today. Under these conditions, a considerable quantity of sand or silt (fine clay) may accompany such organic accumulations. Subject to the type of sediment deposited, a further wide range of coals leading to sedimentary rock types may form. Such sediments are typically massive, exhibiting little or no banding in their lithotypes.
Subject to the quantity of organic matter relative to mineral matter in the mixture, a number of different ‘coally’ sediments may be classified. If the layer contains more maceral than mineral content, i.e. < 50% by mass of ash ad, the deposit would be classified as a coal and categorised into different grades subject to ash content i.e. from low-ash (< 10% ash) to high-ash (30–50% ash) categories of coal.
If, however, the inorganic mineral matter is more prolific in the mix than the organic matter, then, subject to the nature, size and content of minerals contained in the rocks, they may be termed dark grey to black carbonaceous shales, mudstones or siltstones, the degree of colour indicating the amount of organic matter associated within them. In some coal sequences, as for example the Coal Zones in some the Upper Permian Gondwana coalfields, rocks of this kind grade frequently from mineral-rich coally sediments such as siltstones and shales into true coal seams and vice versa over very short vertical distances (millimetres and centimetres). Known colloquially as ‘bar-code’ coals (due to their similarity to bar codes in retail stores), these seams are difficult to sample, analyse and evaluate for marketing purposes. Sampled too widely (incorporating multiple bands), the samples would be categorised as ‘rocks’, given average ash contents of well over 50% ad, but sampled in narrow bands, they are categorised as low to medium ash coals alternating with interbedded carbonaceous shales. It is these complex coally sediments with overall ash contents in excess of 50% that are included in the resources of some coal mines in a number of Gondwana countries. The run-of-mine coals from such seams are washed in various beneficiation processes to yield 12–15% of low-ash coals for high-value markets, a moderate yield of ‘middlings’ products with coals of 35–45% ash on average (including some 60–70% ash products) which are sent to local power stations, and a high yield of very high-ash material which is discarded. This is in stark contrast to the low-ash products produced from the typically low-ash, thick, vitrinite-rich bright seams encountered in many coalfields in the USA and EU (Germany, UK, and Russia).
In areas of relatively wide open water within a coal-swamp, other coally sediments containing macerals and minerals are laid down in the coal-to-sediment continuum. Pollens and spores and leaves that fall during autumn seasons are blown by wind and carried by water into that environment. Where stagnant conditions prevail, algal communities flourish, some of which die, sinking to the bottom to lie amongst the pollens, spores, leaves and fine clay or silt-rich sediments. When found in sufficient quantities, such liptinitic-rich sediments are known as sapropelic coals (deeper water coals). Due to the high ash content of such sediments, they are often overlooked in geological borehole core assessments but, if analysed, they may be found to yield high volatile matter content and valuable oily, waxy and bituminous- like substances, their primary drawback being the relatively high ash content. In some seams, bands of such sapropelic coals are sufficiently thick and organic-rich to warrant extraction for commercial coal-to-liquid purposes. The high volatile matter content and ease of ignition of such coals would explain the conundrum encountered in some mines where it is claimed that the ‘shales or mudstones’ exhibit faster rates of self-heating leading to spontaneous combustion than the associated coals themselves.
Where the spores, pollens and leaf cuticles concentrate in such sapropelic coals, these are specifically known as cannel coals, whereas those specifically enriched in algal matter are termed boghead coals. The inclusion of marine organisms leads to the development of oil shales in the natural sedimentary continuum.
The massive nature of the open water sapropelic coally sediments is in stark contrast to that found in coals accumulating on or close to land which supports terrestrial plants, namely, humic coals. Humic coals are generally stratified with bright bands (vitrinite-rich), often alternating with, or grading into, dull bands. Where bright and dull bands alternate rapidly in narrow layers they are known as banded coals. Sapropelic coals have a characteristic satin lustre, brown streak, and a specific form of breakage (conchoidal, in a circular or curved manner).
Quality distribution
The distribution of coal quality in a coal seam is controlled initially by the nature of the sedimentary environment and related topography under which the peat swamp originally formed. In uneven topography or areas in which peat swamps have high and low-lying areas of accumulation, the decomposition of organic matter will vary at different points – some in wet, some in dry, and others in transitional geochemical environments and sedimentological conditions. Such conditions were typical of the low-lying unevenly-floored swamps that arose in the early Permian coal-forming periods in Gondwanaland after the retreat of glaciation. As a consequence, proportions of macerals and minerals and their resulting microlithotypes and lithotypes changed rapidly over short lateral distances (sometimes within 100 m). This set of circumstances was further affected by proximity to local meandering rivers, which are known sources of sandy and silty sediments. Some river-borne sediments were deposited in pods or parcels in restricted locations within an ancient riverbed. These are now encountered as irregular, in-seam lenses known as ‘stone rolls’, which are known to cause undue wear and tear and severe abrasion during the course of mining when using long and short wall continuous mining machinery. Where flooding has caused rivers to break their banks, thereby carrying loads of sediments over extended areas of the original peat swamp, thicker layers of sediment may be deposited over these areas. This could lead to more substantial rock partings, which would clearly split a coal seam within a sequence of peat accumulation. Such sedimentation could be narrow or thick, and comprise siltstones, mudstones, shales or sandstones, or it could simply lead to mineral-rich low grade coals grading into carbonaceous shales.
These coal quality-affecting factors have been proven to have significant negative commercial impact on the yield and value of coals mined from a number of lower seams in some Gondwana coalfields.
In contrast, seams in the Carboniferous coals of the US and Europe are generally known to be continuous in content and quality for many tens of kilometres without significant change. These arise from the widespread water-logged swamps growing in topical to sub-tropical climates with long growing seasons and moderate to heavy rainfall. Plants extended their geographic coverage and increased in size. A forest could cover 20 000–25 000 square miles in low-lying flat land near shallow seas or rivers. Only occasional drought conditions or flooding affected the peats developing in Laurasian coalfields at that time.
Microlithotypes
Where organic and inorganic constituents of coal combine in various associations to form microscopic layers or bands, these are termed microlithotypes. By definition, these are greater than 50 μm in width. When such microlithotypes are enriched (> 95%) in a single maceral group, they are considered to be a monomaceral microlithotype. Mixed associations of macerals with more than 5% of two maceral groups would be termed a bimaceral microlithotype, and if all three macerals are present, this would be termed a trimaceral microlithotype. The names are linked to the macerals contained in them. For example, a vitrinite-dominated microscopic band or microlithotype is termed vitrite, whereas an inertinite-rich band is entitled inertite. If bands contain two macerals greater than 5%, the microlithotype would be named according to the most common form present, e.g. vitrinertite (vitrinite more common) or inertovitrite (inertinite more common). Where inertodetrinite particles dominate the band, the microlithotype would be termed inertodetrite. Where all three macerals are present in proportions greater than 5%, the band would be termed trimacerite. In cases where minerals are present in significant quantities (e.g. between 25% and 50% by volume for quartz, clays and carbonates, and 5–20% for pyrite), the microlithotype term is carbominerite, and if over 50% mineral matter is present, the term is minerite (i.e. rock in the form of carbonaceous shale, mudstone, siltstone or sandstone). The microlithotype classification is listed in Table 3.4.
Table 3.4
| Microlithotypes | |
| Monomaceral | • Vitrite – > 95% vitrinite • Liptite – > 95% liptinite • Inertite – > 95% inertinite |
| Bimaceral | • Vitrinertite – > 95% vitrinite + inertinite • Clarite – > 95% vitrinite + liptinite • Durite – > 95% liptinite + inertinite |
| Trimaceral | • Duroclarite – all groups > 95%, vitrinite dominant • Clarodurite – all groups > 5%, inertinite dominant • Vitrinertoliptite – all groups > 5%, liptinite dominant |
| Mineral-rich | • Carbominerite – 20–60% (vol) silicates and carbonates Or 5–20% (vol) sulphides • Minerite – > 60% (vol) silicates and carbonates Or > 20% (vol) sulphides |
3.3.2 Abnormal conditions found in coal
A characteristic not often considered when evaluating the value and likely technological performance of a coal, and which is easily identified under a coal petrographic microscope, is the condition of a coal. This has become important in recent years especially in regions where
• surface mining is taking place – which may lead to oxidation and weathering of shallow seams in certain areas of the mine,
• igneous intrusions are abundant – leading to heating and possible burning in situ of the coal, thereby changing the quality of the coal thus affected in the seam,
• groundwater is stored within the seam – this may indicate the presence of an aquifer, which can lead to pressure cracking and brittleness of the coal on handling, as well as unusually excessive and difficult to extract ‘apparent bed moisture’ when evaluating the coal for market purposes,
• rock movements, folding and faulting has occurred – this may lead to an unusually brittle condition and excessive friability on handling such coal, or
• coal has been stockpiled untended and unprotected for some time – this could lead to self-heating, spontaneous combustion and possible burnout of parts of that stockpile, which in turn would affect the quality and performance of the coal when sold to users.
All factors listed above have deleterious impacts on the utilisation of coal.
No standards exist for such petrographic analyses, but a number of coal specialists have developed personal classification systems for the identification of such conditions and these are used on a routine basis for commercial application.
3.4 The impact of petrographic characteristics on the technological properties of coal
In addition to the organic and inorganic components in coal and the conditions to which they have been exposed, a further factor is vital to the understanding of coal and its technological properties: namely, the rank or maturity of coal. Thus section defines the concept of rank and outlines the application of petrography to two aspects of utilisation.
3.4.1 The concept of rank
During the process of biochemical degradation (early peat accumulation), plant material may either be submerged in water with restricted oxygen supply, which results in the production of humic acids and partial or full gelification of the woody plant tissue (peatification), or the plant material may undergo varying levels of oxidation and desiccation when exposed to air to varying degrees. In some cases, putrefaction (fermentation) occurs when plant material is subjected to reducing conditions. This results in anaerobic bacteria consuming the oxygen of organic substances, thereby transforming them into hydrogen-rich products. For example, the fermentation of cellulose leads to the production of hydrogen, methane, acetic acid, carbon dioxide and butyric acid. The coals which form from these processes are generally rich in extractable oils and nitrogen.
Once the accumulating plant matter is buried at depths below 1 m, biochemical degradation ceases to function and geochemical coalification takes place. During coalification, chemico-physical changes occur over time and under conditions of higher temperature and pressure, thereby giving rise to the process of maturation and metamorphosis (changes) in the various plant fragments (macerals). This results in the transformation of the original peat swamp plant materials through progressive stages of brown coal (lignite), sub-bituminous and bituminous coals to anthracite and meta-anthracite.The end-point of this continuum is graphite, which occurs only rarely in nature as a result of intense pressure and temperature in some coalfields of the world. The level to which a coal has reached in this coalification series is termed its rank or level of maturity. Chapter 2 describes this process in further detail.
The earlier process of peatification which resulted in the breakdown of plant matter into organic fragments in the original peat swamp (thereby controlling the proportions of maceral forms) is entirely independent of the maturing process or increase in rank.
The maceral groups, once formed, undergo different and varying levels of progressive physical, chemical and optical (microscopic) changes with increasing rank but their proportions and shape, with some exceptions, remain the same. In general, overall changes with rank are considered to take the form of loss of moisture and volatile matter with the consequent increase in carbon content and aromaticity. However, this is not necessarily the case in all coalfields.
As the inert forms of inertinite group macerals are highly aromatic from source in the peat stage, and therefore have little or no volatile matter to release, there is no discernible change with increasing rank. Their properties – aromaticity, carbon content, size and shape – remain the same through the entire rank process. Reactive inertinite macerals, on the other hand, change slightly with a limited loss of volatile matter and minor colour changes. Liptinite, in stark contrast, changes rapidly with increasing rank, first emitting high quantities of volatile matter from its abundant aliphatic chains in the early bituminous ranges of rank (bituminous D), followed by heavier hydrocarbons, waxes and tarry substances in early mid bituminous levels of rank (bituminous C). Thereafter, in the later mid bituminous range of rank (bituminous B to A), these macerals completely cease to exist, disappearing from view and merging into vitrinite. The use of liptinite is therefore as limited as inertinite in assessing the rank of coal when volatile matter or carbon content are used as rank-defining parameters.
In the case of the vitrinite maceral group, however, the loss of volatile matter and increase in carbon content has been shown to change in constant and linear manner with the increase of rank. Thus, as the coals of the Carboniferous period in Laurasia possess high vitrinite content (> 75%), volatile matter and carbon content have long been considered the definitive parameters indicative of the level of rank reached by a coal. In the Gondwana coalfields, however, where vitrinite varies from 0% to 75%, with an average of 25% for seams in that region, and as the inertinite group macerals with low and variable volatile matter contents dominate the coals, this is not necessarily the case.
In short, volatile matter and carbon content in Gondwana coals are not indicative of the true rank or maturity of a coal (unless containing high vitrinite content). In this coal-forming region, volatile matter and carbon content simply present values to which highly variable and unknown maceral proportions (some high and others low in volatile and carbon content), rank and condition all contribute. Factors such as heat effect by intrusive igneous sills and dykes, and weathering or oxidation of shallow seams superimpose further complications upon volatile matter and carbon contents, thereby once again disaffecting a true interpretation of those analytical parameters. It is possible, for example, to have a coal with a volatile matter content of 16% ad which may appear to be a lean low volatile bituminous coal (or bituminous A), but in fact, based upon true determination of rank, is found to be a sub-bituminous coal with high proportions of oxidised and inert inertinite macerals. Such ‘mis’interpretations have serious practical and technological implications for such coals.
During the 1970s the need to refine a method for the true identification of rank on an international basis was initiated by the Coal Working Party of the UN ECE, given that the international trade in coal was expanding at that time. Results of investigations proved that increasing rank changed linearly in parallel with the proportion of light emanating from the surface of vitrinite particles. In the lignitic and sub-bituminous level of rank, vitrinite reflects medium to dark grey relative to normal white light. With increasing levels of rank, vitrinite passes through changing levels of grey, becoming increasingly lighter and whiter as it reaches its ultimate level of bright white in the meta-anthracite and graphite categories. Thus, based upon the regularity of this change, the reflectance of vitrinite values as defined in ISO 11760 (2005) was drawn up and designated as the official international method for the definition of the rank in the hard or black coal range (bituminous and anthracite), irrespective of the coal’s origin or maceral composition. Rank in the lignitic or sub-bituminous range of rank remains defined according to moisture level or heat content.
The reflectance of vitrinite is now reported as the conventional method of determination of rank, using the mean value of the quantity of light as statistically measured petrographically on the surface of specific number of vitrinite particles per sample. The value is reported as a percentage relative to white light, and either as a random value (Vr% random) or maximum value (Vm% maximum). The distribution of the values for a specific coal is portrayed as a histogram, indicating the range of values seen for a specific coal. Standard deviation values indicate the mode of distribution (single seam or blend with coals of several ranks). The internationally accepted categories of rank as defined by vitrinite reflectance (mean random) are presented in Table 2.6 in Chapter 2.
3.4.2 The impact of rank and maceral composition on coal utilisation
The rank and maceral composition of a coal have direct and relevant significance to the technological use of that coal. This includes five forms of reactivity in commercial operations, including reactivity to combustion, gasification, reduction in the metallurgical industry, hydrogenation/liquefaction and self-heating leading to spontaneous combustion. As these factors will be discussed in further chapters, only a summary of two areas of utilisation will be provided here.
Combustion and power generation
Inert forms of inertinite macerals at any level of rank will not ignite or combust easily without high temperatures and higher than normal excess (stoichiometric) oxygen levels. Highly oxidised forms will pass through a major pulverised fuel boiler without ever changing or being consumed. Vitrinite, however, in the bituminous range of rank will initiate devolatilisation at increasing temperatures with increasing levels of rank. Softening and swelling of the devolatilising particles occur simultaneously and, once all volatiles have been emitted, the resulting ‘char’ (devolatilised coal particle) hardens in its new porous form. The degree of porosity and thickness of char walls vary with rank, with highest porosity and thinnest walls in low rank bituminous coals (bituminous D) and the least porosity with little or no change in wall structure in the anthracitic level of ranks. Such structures have considerable impact on combustion. Vitrinites in the lower ranks of bituminous coal (C and D) are the most reactive in combustion, devolatilising easily and igniting and burning out at lower temperatures relative to higher ranking coals. The higher ranking coals initiate devolatilisation at considerably higher temperatures, are more difficult to ignite (fewer volatiles) and take longer to burn out, given their increased carbon content and aromaticity. Such coals may require specific conditions to effect ignition. Mixing or blending of coals at different ends of the rank scale can result in poor combustion, as the low rank coals may ignite and burn out before the high rank coals have reached their temperature of devolatilisation.
The role of minerals in combustion is equally complex and requires indepth evaluation. All minerals have the capacity to soften and melt, subject to temperature and gaseous environment, some occurring at lower temperatures relative to others. This phenomenon causes slagging on boiler walls and superheaters, and fouling on backend tubing. Softening and melting may also be induced in boilers in the presence of reducing environments (i.e. with limited air or oxygen availability). Such conditions occur most typically when burning coals with high proportions of inert inertinite macerals, which typically consume higher than normal quantities of oxygen (well above calculated stoichiometric plus excess air levels) and which combust at higher temperatures. Recent evidence has proved that some inertinite-rich coals combust at temperatures as high as almost 1800 °C, suggesting that coals with apparently acceptable, analytically reported ash fusion temperatures of 1400–1500 °C may be expected to induce slagging under such conditions.
Some minerals also emit incombustible volatiles which, if such the minerals were present in high enough quantities, can lead to the quenching of flames in a boiler. Clays, for example, emit moisture from their water of crystallinity, losing approximately one third of their mass in the process. Similarly, carbonates emit CO2, losing about 45% of their mass in the process. Both minerals devolatilise at temperatures below 1000 °C (carbonates from 650 °C to 700 °C), indicating that such incombustible gases are captured in conventional volatile matter measurements for proximate analyses. In cases of high-ash coals (above 30% ash content), as much as one third of the volatiles reporting to volatile matter content in a proximate analysis may be of incombustible origin.
Calculations of ash fusion temperatures and related ash properties are conventionally undertaken by the use of chemical formulae such as the acid/base ratio using oxides of various inorganic elements, e.g. SiO2, Al2O3, Fe2O3, and others. It is pertinent to note, however, that these inorganic elements are found in a number of different minerals, and that it is the minerals that dictate the technological performance of the ‘ash’ in coal, not simply the elements themselves. For example, Fe2O3 is usually associated with SO3, indicating the presence of pyrite (the Fe sulphide mineral responsible for emitting SOx); however, Fe is also present in certain carbonate minerals which have no relevance to sulphur in coal or the properties of pyrite. It is for these reasons that the prediction of ash fusion temperatures using simple commonly used chemical oxide ratios is not necessarily accurate in cases where mineral proportions differ from a known norm. This situation supports the case for the use of more advanced mineral identifications, such as CCSEM, for evaluating a coal more confidently for commercial trade and utilisation purposes.
The petrographic characteristics of coal as applied to combustion apply equally well to those required in gasification. Char forms may differ slightly in some gasification processes, and the environment for optimum reaction may require different atmospheric conditions.
Coke for the metallurgical industry
Coke is manufactured from prime coking coal, baked in coke ovens, and used as a valuable reductant in the metallurgical industry. The coal required for these purposes is a vitrinite-rich coal with a certain proportion of inertinite (to add strength to the walls of the coke); it is required to have low ash content (< 12%), low sulphur (< 1%) and phosphorus content (< 0.001%) and – most importantly – requires a coal with a maturity that falls in the mid bituminous level of rank (bituminous B). At this level of rank in the coalification (maturing) process, vitrinite has matured to a point where reordering of the aromatic and aliphatic molecules has taken place in a manner such that, on heating under specific conditions, there is sufficient volatile matter to create a char with the correct degree of porosity (important for gaseous permeability in the blast furnace), walls thick enough to provide strength to support the burden in a blast furnace, and sufficient molecular realignment to provide a crystalline texture in the walls to allow for good reduction in the liquid metal phase in a submerged arc furnace.
Coking coal of the type required above is limited in availability and expensive in the world today, so the blending of coals with ranks in close proximity to the prime level of rank, and those with varying maceral proportions, is now being practised in metallurgical operations the world over. However, this is a highly complex and scientific operation based largely on petrographic characteristics supported by key physical and chemical tests in order to provide the properties required by industry.
Currently, research is under way to find alternative coal or carbon-based sources with the correct metallurgical properties in order to substitute those materials in place of coke as reductants in the metallurgical industry. Such materials include chars in various forms, heat treated briquetted materials, and high rank coals with specific properties. All are evaluated using petrography with supporting physical tests in order to evaluate and compare their efficiencies in the various metallurgical processes.
3.5 Development of modern techniques in coal petrographic analysis
In the early years of coal investigations, analysis of the constituents of coal was undertaken by cutting ultra-thin sections of coal from hand specimens and examining them microscopically using transmitted light. This was laborious and difficult and did not indicate the quality of coal in a seam, stockpile or shipment. During the Second World War new techniques were developed, using crushed coal, embedding the particles of coal in a resinbound pellet, polishing the surface, and examining it using reflected light and through emersion oil. This system has developed to an advanced degree, to the extent that currently the primary method of analysing coal to determine its organic constituents (macerals, microlithotypes, rank and condition) is by analysing such coal grains using specially designed, highly technical petro- graphic microscopes linked to computer databases. Rank is determined by measuring the level of reflectance, using a highly sensitive photomultiplier attached to the reflectance microscope and computer system.
Samples of coal may now be drawn from any source and be considered as a representative sample sent for chemical analyses for commercial purposes. While blocks of hand specimens of coal may be examined if specific geological features are required, more conventionally coal is crushed to − 250 μm. A portion of the crushed coal, now in powder form, is mixed with epoxy resin and set.
The pellets of crushed coal are then subjected to a series of increasingly fine grinding stages till a final 0.5 μm polish on silk. The pellets are examined using white light and blue-light excitation at a combined magnification of between 250 and 500 using reflected-light oil-immersion objectives on reflecting ore microscopes. Reflectance of vitrinite for rank determination is undertaken using a highly controlled and regulated photomultiplier light-reader with a digital readout. The data is entered into a computer for later print-outs. Results are produced in statistical format reports, with tabulated distributions, histograms and with standard deviation analyses.
Maceral distribution is undertaken on a statistical basis using a specific point counting system while microlithotype analyses are undertaken using a 50 μm grid and counts based on the statistically required number of fields of view. Conditional assessments of a coal are undertaken on a similar basis to microlithotype analyses with categories provided for oxidised, weathered, heat-affected, burnt (by igneous intrusion or spontaneous combustion) or groundwater-ridden conditions. Reflectance measurements are conducted on telocollinite forms of vitrinite.
Attempts to automate petrographic analyses have been made on a number of occasions over the recent years, and indeed such systems are being used in some commercial laboratories today. The approach is based on the fact that liptinite is always darker and greyer than vitrinite, which in turn is always darker and greyer than inertinite. When simple fresh coals from single seams or washed products are supplied for analysis, this approach is acceptable. However, when coals have been oxidised, heat affected or blended with coals of different ranks, this system cannot distinguish these factors, and overlaps in reflecting categories for quite different reasons can occur. This ultimately gives incorrect readings. In such cases, only well- trained man-powered operator investigation can diagnose the anomalies.
Attempts have also been made to use CCSEM or QEMSCAN for organic matter analysis in coal. Whilst normally used for mineral matter identification and mineral matter associations, this powerful tool has been able to establish C, H and O in the organic matter of a coal sample. Based on the ratios of C, H and O, attempts have been made to predict the maceral composition of coal, using formulae similar to those published by Seyler in the 1930s. This has, in some cases, been successful when coals of similar type, grade and rank have been used. Once again, however, if the coals have been exposed to unusual conditions or have been mixed with different ranks, a situation similar to that described in the paragraph above can occur and the results shown to be anomalous. Furthermore, work undertaken by Snyman in 1995 has shown that the Seyler diagram has had to be adapted to a considerable extent for use in Gondwana coals, due to the presence of the highly variable inertinite macerals. It is difficult to achieve acceptable levels of interpretation when analysing variable coals. This approach is therefore limited to analyses of known, simple and similar types and grades of coal.
The analyses of mineral matter and its associations in coal can be undertaken using the advanced technologies of CCSEM or QEMSCAN supported by related analytical techniques including XRD and XRF (O’Brien et al., 2007). The significance of CCSEM or QEMSCAN is the ability of this type of equipment to determine the degree of intergrowth and levels of association of organic and inorganic matter whilst also determining the nature, size and chemical composition of the mineral matter. This has most value in predicting the potential degree of liberation of minerals from organic material when assessing the washability of coal for commercial purposes. Similarly, this can be used for monitoring the transformation of minerals into ash during combustion and gasification processes, and in so doing, determining the cause of slagging or fouling in a boiler.
Recent research undertaken by O’Brien et al. (2011) has developed organic and inorganic microscopy even further, this time by establishing that maceral reflectance can now be measured as effectively in air as under oil immersion and that, linked with SEM-based equipment for mineral identification, a fully integrated optical imaging system using both systems may be possible. Rather than the traditional analyses of coal using two different pieces of equipment, this combined and integrated system is likely to lead to fast and effective collection of both organic and inorganic data using both systems in one piece of equipment taken from the same coal pellet surface. This bodes well for more cost-effective and yet advanced assessments of coal for trade and utilisation in future.
3.6 Conclusion
The understanding of coal and its constituents has come a long way since the start of investigations in the 1920s. Through petrographic and CCSEM and QEMSCAN analyses, much can be predicted with respect to the technical performance of a coal, its ability to be upgraded, and its capacity to be used for specific and high-value purposes. In the words of one sage employed in a metallurgical company, if I was to be given one and only one analysis with which to select the coals best suited for my purposes [coke making], I would choose petrography. Thirty years later, the same words still apply.
3.7 Bibliography
Falcon, R.M.S., A brief review of the origin, formation and distribution of coal in Southern AfricaAnhaeusser, Maske, eds. Mineral Deposits of Southern Africa, 1986. [Geological Society of SA].
Falcon, R.M.S., Snyman, C.P., An introduction to coal petrography: Atlas of petrographic constituents in the bituminous coals of Southern Africa. Review Paper no 2. 1986. [February 1986, Geological Society pf SA].
Holland, M.J., Cadle, A.B., Pinheiro, R., Falcon, R. Depositional Environments and Coal Petrography of the Permian Karoo sequence: Witbank Coalfields, South Africa. March. International Journal of Coal Geology. 1989; 11(2):143–169.
O’Brien, G., Jenkins, B., Ofori, P., Ferguson, K. Semi-automated petrographic assessment of coal by coal grain analysis. Minerals Engineering. 2007; 20:428–434.
O’Brien, G., Gu, Y., Adair, B.J.I., Firth, B. The use of optical reflected light and SEM imaging systems to provide quantitative coal characterisation. Minerals Engineering. 2011; 24:1299–1304.
Oboirien, B.O., Engelbrecht, A.D., North, B.C., Falcon, R. Mineral-char interaction during gasification of high ash coals in fluidised bed gasification. Energy and Fuels. 2011; 25:5189–5199.
Stach, E., Mackowsky, M.-T.h., Teichmuller, M., Taylor, G.H., Chandra, D., Teichmuller, R. Stach’s Textbook of Coal Petrology. Berlin: Gebruder Borntraeger; 1982.
Taylor, G.H., Teichmuller, M., Davis, A., Diessel, C., Littke, R., Robert, P. Organic Petrology. Berlin: Gebruder Borntraeger; 1998.
