5.2.1 Batch Reaction Times
The time necessary to achieve a specific conversion depends upon how fast the reaction takes place, which in turn is dependent on the rate constant and the reactant concentration. To get a feel of how long it takes to carry a batch reaction, we shall calculate the batch reaction times for different values of the reaction rate constant, k, for a first- and for a second-order reaction. First, let’s solve for the time to achieve a conversion X for the second order reaction
2A → B + C
The Algorithm
- The mole balance on a constant-volume, V = V0, batch reactor is
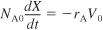
Dividing by NA0 and recognizing CA0 = NA0/V0 we obtain
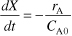
- The rate law is
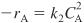
- From stoichiometry for a constant-volume batch reactor, we obtain

- Combining the mole balance, rate law, and stoichiometry we obtain

- To evaluate, we separate the variables and integrate

Initially, if t = 0, then X = 0. If the reaction is carried out isothermally, k will be constant; we can integrate this equation (see Appendix A.1 for a table of integrals used in CRE applications) to obtain

This time is the reaction time t (i.e., tR) needed to achieve a conversion X for a second-order reaction in a batch reactor. In a similar fashion, we can apply the CRE algorithm to a first order reaction to obtain the reaction time, tR, needed to achieve a conversion X
![]()
It is important to have a grasp of the order of magnitudes of batch reaction times, tR, to achieve a given conversion, say 90%, for different values of the product of specific reaction rate, k, and initial concentration, CA0. Table 5-1 shows the algorithm to find the batch reaction times, tR, for both first- and a second-order reactions carried out isothermally. We can obtain these estimates of tR by considering the first- and second-order irreversible reactions of the form
2A → B + C
Table 5-1. Algorithm to Estimate Reaction Times

For first-order reactions the reaction time to reach 90% conversion (i.e., X = 0.9) in a constant-volume batch reactor scales as
![]()
If k1 = 10–4 s–1,
![]()
The time necessary to achieve 90% conversion in a batch reactor for an irreversible first-order reaction in which the specific reaction rate, k1, is (10–4 s–1) is 6.4 h.
For second-order reactions, we have
![]()
If k2CA0 = 10–3 s–1,
![]()
We note that if 99% conversion had been required for this value of kCA0, the reaction time, tR, would jump to 27.5 h.
Table 5-2 gives the order of magnitude of time to achieve 90% conversion for first- and second-order irreversible batch reactions. Flow reactors would be used for reactions with characteristic reaction times, tR, of minutes or less.

Estimating Reaction Times
Table 5-2. Batch Reaction Times

The times in Table 5-2 are the reaction time to achieve 90% conversion (i.e., to reduce the concentration from CA0 to 0.1 CA0). The total cycle time in any batch operation is considerably longer than the reaction time, tR, as one must account for the time necessary to fill (tf) and heat (te) the reactor together with the time necessary to clean the reactor between batches, tc. In some cases, the reaction time calculated from Equations (5-4) and (5-5) may be only a small fraction of the total cycle time, tt.
tt = tf + te + tc + tR
Typical cycle times for a batch polymerization process are shown in Table 5-3. Batch polymerization reaction times may vary between 5 and 60 hours. Clearly, decreasing the reaction time with a 60-hour reaction is a critical problem. As the reaction time is reduced (e.g., 2.5 h for a second-order reaction with k2CA0 = 10–3 s–1), it becomes important to use large lines and pumps to achieve rapid transfers and to utilize efficient sequencing to minimize the cycle time.
Table 5-3. Typical Cycle Time for a Batch Polymerization Process

Usually one has to optimize the reaction time with the processing times listed in Table 5-3 to produce the maximum number of batches (i.e., pounds of product) in a day. See Problems P5-8(e) and P5-12(e).
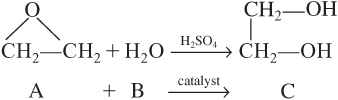
In the next four examples, we will describe the various reactors needed to produce 200 million pounds per year of ethylene glycol from a feedstock of ethane. We begin by finding the rate constant, k, for the hydrolysis of ethylene oxide to form ethylene glycol.
Example 5-1. Determining k from Batch Data
It is desired to design a CSTR to produce 200 million pounds of ethylene glycol per year by hydrolyzing ethylene oxide. However, before the design can be carried out, it is necessary to perform and analyze a batch reactor experiment to determine the specific reaction rate constant, k. Because the reaction will be carried out isothermally, the specific reaction rate will need to be determined only at the reaction temperature of the CSTR. At high temperatures there is a significant by-product formation, while at temperatures below 40°C the reaction does not proceed at a significant rate; consequently, a temperature of 55°C has been chosen. Because water is present in excess, its concentration (55.5 mol/dm3) may be considered constant during the course of the reaction. The reaction is first-order in ethylene oxide.
In the laboratory experiment, 500 mL of a 2 M solution (2 kmol/m3) of ethylene oxide in water was mixed with 500 mL of water containing 0.9 wt % sulfuric acid, which is a catalyst. The temperature was maintained at 55°C. The concentration of ethylene glycol was recorded as a function of time (Table E5-1.1).
a. Derive an equation for the concentration of ethylene glycol as a function of time.
b. Rearrange the equation derived in (a) to obtain a linear plot of a function concentration versus time.
c. Using the data in Table E5-1.1, determine the specific reaction rate at 55°C.
Table E5-1.1. Concentration-Time Data

Part (a)
- The mole balance given in Equation (1-5) for a constant volume, V0, well-mixed batch reactor can be written as


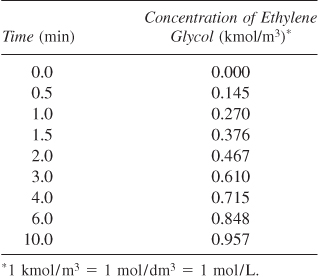
Taking V0 inside the differential and recalling that the concentration is

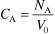
then the differential mole balance becomes

- The rate law is


Because water is present in such excess, the concentration of water at any time t is virtually the same as the initial concentration, and the rate law is independent of the concentration of H2O. (CB ≅ CB0.)
- Stoichiometry. Liquid phase, no volume change, V = V0 (Table E5-1.2):
Table E5-1.2. Stoichiometric Table


Recall that ΘB is the ratio of the initial number of moles of B to A (i.e.,
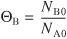 ).
).For species B
CB = CA0(ΘB – X)
We quickly see that water is in excess, as the molarity of water is 55 moles per liter. The initial concentration of A after mixing the two volumes together is 1 molar. Therefore,

The maximum value of X is 1, and ΘB>>1, therefore CB is virtually constant
CB ≡ CA0Θ = CB0
For species C the concentration is
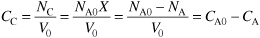
- Combining the rate law and the mole balance, we have


- Evaluate. For isothermal operation, k is constant, so we can integrate this equation (E5-1.5)
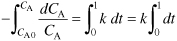
using the initial condition that when t = 0, then CA = CA0 = 1 mol/dm3 = 1 kmol/m3.
Integrating yields
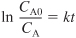
The concentration of ethylene oxide at any time t is

Part (b)
The concentration of ethylene glycol at any time t can be obtained from the reaction stoichiometry:
![]()
Rearranging and taking the logarithm of both sides yields
![]()
Part (c)
We see that a plot of ln[(CA0 – CC)/CA0] as a function of t will be a straight line with a slope = k. Using Table E5-1.1, we can construct Table E5-1.3 and use Excel to plot ln(CA0 – CC)/CA0 as a function of t.
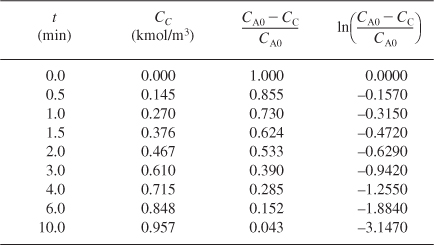
From the slope of a plot of ln[(CA0 = CC)/CA0] versus t, we can find k, as shown on the Excel Figure E5-1.1.
Figure E5-1.1. Excel plot of data.
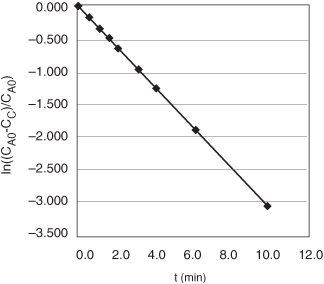
Slope = –k = –0.311 min–1
k = 0.311 min–1
The rate law becomes
–rA = 0.311 min–1CA

The rate law can now be used in the design of an industrial CSTR. For those who prefer to find k using semilog graph paper, this type of analysis is given in the Chapter 7 Summary Notes on the DVD-ROM. An Excel tutorial to calculate slopes for semilog plots is also given in the Summary Notes for Chapter 3. More details on the solution algorithm can be found at the URL: www.engin.umich.edu/~problemsolving.
Analysis: In this example we used our CRE algorithm
(mole balance → rate law → stoichiometry → combine)
to calculate the concentration of species C, CC, as a function of time, t. We then used experimental batch data of CC versus t to verify the reaction as a first-order reaction and to determine the specific reaction rate constant k.
5.3 Continuous Stirred Tank Reactors (CSTRs)
Continuous stirred tank reactors (CSTRs), such as the one shown here schematically, are typically used for liquid-phase reactions.

In Chapter 2, we derived the following design equation for a CSTR:
![]()
which gives the volume V necessary to achieve a conversion X. As we saw in Chapter 2, the space time, τ, is a characteristic time of a reactor. To obtain the space time, τ, as a function of conversion, we first substitute for FA0 = υ0CA0 in Equation (2-13)
![]()
and then divide by υ0 to obtain the space time, τ, to achieve a conversion X in a CSTR
![]()
This equation applies to a single CSTR or to the first reactor of CSTRs connected in series.