3
Atom: Quarks, Protons, and Electrons
3.1 Introduction
In this chapter, we will make a brief introduction about the physics behind electricity and electromagnetism, starting at the atomic level.
3.2 Atoms and Quarks
The word atom comes from ancient Greek word meaning “indivisible,” because it was believed at the time that the atom was the smallest part of matter and could not be divided.
At the atomic level, atoms are composed of protons, neutrons, and electrons.
Today we know that these elements are composed of more elementary elements called quarks, and known to exist in six variations called flavors: top, bottom, charm, strange, up, and down.
As far as physicists know today, quarks are themselves elements that cannot be divided. Every time you try to divide them, a new quark is created.
Protons for example, are composed of two up quarks and one down quark, and neutrons are composed of two down quarks and one up quark (Figure 3.1).
Protons have a positive electric charge and neutrons have no electric charge.
In terms of reference charge, a proton is said to have an electric charge equal to 1. This charge, generally referred as e+, is equal to 1.602176634 × 10−19C.1

Figure 3.1 A proton and its quarks.
3.3 Electrons
Electrons are stable subatomic particles with a negative charge that can be found in all atoms and act like charge carriers.
In terms of reference charge, an electron is said to have a charge equal to −1 or, in other words, a charge opposite to the charge of a proton. For that reason, its charge, generally referred as e−, is equal to −1.602176634 × 10−19C.
In the early days, science pictured electrons spinning around atomic nucleus like planets spinning around the sun. Today we know that this image is false. Electrons oscillate frenetically around the atomic nucleus, and their position cannot be determined,2 like they were in all positions at the same time, a kind of “energetic” cloud.
The idea of an electron orbiting an atomic nucleus like a planet around a sun is wrong because it makes us think about electrons as a solid and discrete element, like a particle. In real life, electrons behave both as particles and waves.3
Physicists knows that electrons occupy orbits around the nucleus at a certain distance, like they were shells. Each orbit is at a discrete level of energy and can only be occupied by electrons having that particular level of energy. If an electron gains energy, it may pass to a superior orbit. If it loses energy, it will decay to a lower orbit and release the extra energy by emitting a photon.4

Figure 3.2 Atom representation.
A representation of an atom is shown in Figure 3.2. The spheres at the center represent the nucleus, nothing more than an agglomeration of protons and neutrons. The circles represent the energetic orbits. Electrons are shown in the diagram as “e” inside the orbits but in real life they can be at any point and in all points at the same time inside a particular orbit.
3.4 Strong Force and Weak Force
Atoms and their protons and neutrons are kept together by powerful attraction forces.
The laws of physics say that elements with the same electric charge repel each other and that elements with opposite electric charge attract each other.
Atomic nuclei may be formed by several protons and neutrons. These protons could never be kept together with other protons, at the required close proximity, to form these atomic nuclei, simply because they would repel each other. A bigger force must exist to overpower the electric repulsion force. This force is called the strong nuclear force or strong interaction.
At a closer range, the strong force is hundreds of times as strong as electromagnetism, a million times as strong as the weak force, and a thousand times as strong as gravitation. The strong force keeps most ordinary matter together and binds neutrons and protons to create atomic nuclei.
The weak force, weak nuclear force or weak interaction, takes place only at very small, subatomic distances, less than the diameter of a proton. It is one of the four known fundamental interactions of nature, alongside the strong interaction, electromagnetism, and gravitation.
This weak force is responsible for radioactive decay and thus plays an essential role in nuclear fission.
3.5 Conductors and Electricity
As mentioned before, atoms are composed of protons, holding a positive electric charge and neutrons that have no electric charge. Electrons are found frenetically oscillating in a cloud of energy around this agglomerate of protons and neutrons.
We know that electrons are charge carriers and that as they move inside a material, they transport electric charges around. This movement of charges is called electric current.
In order for an electron to be able to move around a material, it is necessary, for the material itself, to have a certain kind of atomic structure.
Hydrogen (H), for example, is the simplest atomic element and for that reason the most abundant element in the universe. Hydrogen's nucleus is formed of a single proton and one electron is found orbiting around (see Figure 3.3).
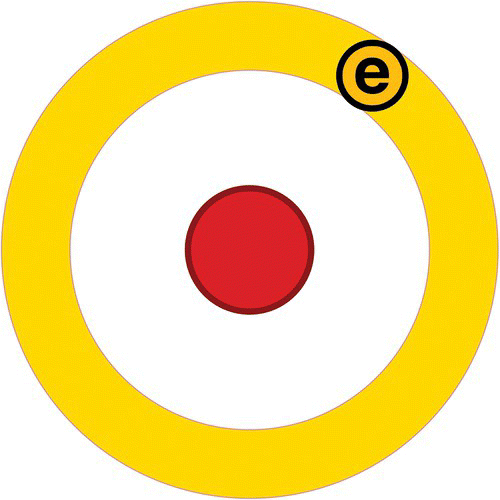
Figure 3.3 Hydrogen atom.
Helium (He), on the other hand, is composed of two protons, two neutrons, and two electrons, like shown in Figure 3.4.

Figure 3.4 Helium atom.
Hydrogen and helium are not good conductors of electricity. They have very few electrons, and these electrons are closer to the nucleus, subjected to the strong force, and, for that reason, are very difficult to move out of the atomic structure.
Copper (Cu), for example, an excellent conductor of electricity, is much more complex than hydrogen and helium. Its nucleus contains 29 protons, 35 neutrons, and 29 electrons. The electrons are distributed in several energetic shells, as represented in Figure 3.5.

Figure 3.5 Copper atom.
3.6 The Shells
Atoms that have a lot of electrons have these electrons distributed through several orbits, or shells, around the nucleus, like shown in Figure 3.5.
An electron needs a certain level of energy to occupy a particular orbit. Orbits that require more energy are farthest from the nucleus.
Each orbit, or shell, allows a certain limited number of electrons. The first six shells, for example, known by the letters K, L, M, N, O, and P, can have 2, 2, 18, 32, 50, and 72 electrons, respectively. The outermost shell of an atom is known as the valence shell.
Electrons are not allowed to exist between shells. If an electron is on a particular shell and absorbs the required energy level to jump to an outer shell, it does by disappearing from the current shell and appearing on the outer shell.
If it is the other way around, that is, an electron is on an outer shell and loses energy to be on a vacant position in an inner shell, it disappears from the current shell and appears on the inner shell. In the process, it releases the extra energy by emitting a photon.
Electrons that are on the outermost shells are less subject to the strong force, exerted by the nucleus. Hence, these electrons can be more freely moved, if energy is applied.
Imagine a block of copper, containing millions and millions of atoms of this element. When these atoms are packed together, a magic happens and something known as a metallic bonding is formed, leaving a huge number of electrons detached from the nucleus. These electrons are now completely free to move if energy is applied.
3.7 Electric Potential
Like explained before, protons have positive electric charge and electrons have negative electric charge.
A large number of electrons on one side of a given material compared with another represent a difference in electric potential between the two points. This difference is electric potential energy stored in the form of electric charge.
A difference in electric potential between two points will create an electric field, and this field will force electrons to move into a specific direction.
The bigger the difference in potential, the bigger is the electric field, and, consequently, the bigger the number of electrons moving, the bigger is the current. A large difference in potential will also cause electrons to move more aggressively through the field.
The difference in electric potential is a kind of potential energy and as an energy it can create work.
The electric potential energy can be defined as the work, in Joules, to move electrons on an electric field.
The difference in electric potential, also known as electromotive force, is measured in Volts5 and is represented by the uppercase letter V in the SI.6
3.8 Current
Like mentioned previously, an electric field applied to a material will create a kind of pressure that will force the electric field to be transmitted from electron to electron at a speed about 63% of the light speed.
The electrons themselves move very slowly, from the lower potential point to the higher potential point, at a speed, called “drift velocity” that is something like 1.5 cm/min. The movement is irregular, like in a random zigzag motion, as they collide with atoms in the conductor.
The effect of having the electric field transmitted at a speed higher than the electron speed can be illustrated, roughly, to what happens on the Newton cradle toy (see Figure 3.6).
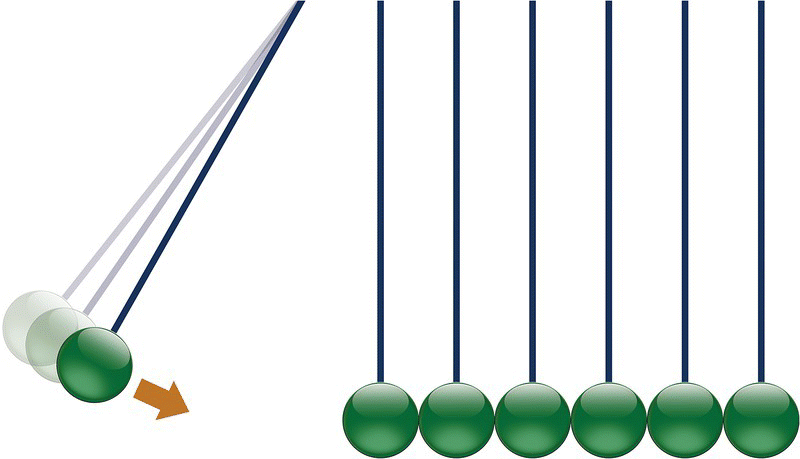
Figure 3.6 Newton cradle.
All balls are at initially at rest until we apply a speed to the first one in the left and make it collide with the other ones. As soon as the collision happens, the last ball in the right jumps. In other words, the balls practically do not move, but the force is transmitted from one to the other through the entire chain.
Current is measured in Ampere7 and is represented by the uppercase letter A in the SI.
3.9 Electric Resistance
Free electrons inside a material can move forced by an electric field, but not all electrons are completely free to move, due to restrictions imposed by the material's structure. These restrictions prevent the free movement of electrons and resist their passage, generating friction and heat.
This restriction is called electric resistance, measured in Ohm,8 and is represented by the Greek letter omega (Ω) in the SI.
Notes
- 1 This is the new value revised by the SI in 2018. The old value was
 .
. - 2 Heisenberg’s uncertainty principle.
- 3 The famous wave–particle duality from quantum mechanics, demonstrated brilliantly in 1802 by Thomas Young, for the first time, by the double‐slit experiment.
- 4 The photon is a type of elementary particle, the quantum of the electromagnetic field including electromagnetic radiation such as light, and the force carrier for the electromagnetic force. The photon has zero rest mass and always moves at the speed of light within a vacuum (source Wikipedia).
- 5 In honor of Alessandro Giuseppe Antonio Anastasio Volta, simply known as Alessandro Volta (1745–1827), an Italian physicist, a chemist, and a pioneer of electricity and power who is also credited as the inventor of the electric battery.
- 6 French's Système Internationale d'Unités (International System of Unities), abbreviated as SI in all languages.
- 7 In honor of André‐Marie Ampère (1775–1836), French mathematician and physicist, credited as the father of electrodynamics.
- 8 In honor of Georg Simon Ohm (1787–1854), German physicist and mathematician.
