Solutions
Publisher Summary
This chapter focuses on the solutions and types of solutions available. A solution is formed when one substance, the solute, is dispersed in a different substance, the solvent, which is present in the largest amount. The substances can be gases, liquids, and solids, and solutions can be composed of any combination of these substances. A true solution is one that is completely homogeneous, that is the same throughout, and that can be separated perfectly into the constituent parts of the solution. A saturated solution is formed when no more solute will dissolve in a fixed volume of solvent at a constant temperature. The easiest way to obtain a saturated solution is to dissolve the solute in the solvent until the excess solute is present and then to filter the solution. A super saturated solution is one that contains more dissolved solute than is needed to form a saturated solution at the sametemperature. A colloidal solution is formed when a disperse phase of particles that are between one nm and 100 nm diameter are distributed in a dispersion medium.
1. A solution is formed when one substance, the solute, is dispersed in a different substance, the solvent, which is present in the largest amount. The substances can be gases, liquids and solids and solutions can be composed of any combination of these as shown in Table 43.1.
Table 43.1
| Solution | Examples |
| Gas in gas | Air |
| Gas in liquid | Lemonade |
| Liquid in liquid | Whisky |
| Solid in liquid | Salt solution |
| Solid in solid | Metal alloys |
2. A true solution is one which is completely homogeneous (i.e. the same throughout) and which can be separated perfectly into the constituent parts of the solution. The relative amounts of solute and solvent present in a solution can be expressed in a number of ways:
(a) Percentage by mass. This is the number of grams of solute in 100 grams of solvent. For example, a 1% sugar solution contains 1 g of sugar in 100 g of water, or alternatively, 1 kg of sugar in 100 kg of water.
(b) Grams per cubic decimetre. The cubic decimetre is one of the traditional volumes of scientific measurement, the litre. The composition of the solution in these terms is the number of grams of solute in 1 cubic decimetre of solvent, i.e. g dm−3. Alternatively, the units used could be the number of kilograms in 1 cubic metre, i.e. kg m−3.
(c) Moles per cubic decimetre. This is the most popular method of expressing the composition in terms of the number of moles of solute in 1 cubic decimetre of solution. This means that the amount of solvent used depends upon the mass of solute used. In chemical terms it means that the actual number of molecules contained in any given volume of the solution is known. For example, a 0.1 M solution (one tenth molar) contains 0.1 moles of solute in 1 cubic decimetre of solution, or 6.023 × 1023 molecules of solute in 1 dm3 of solution.
3. When a solution is formed, the components must be compatible with each other. Many examples are found in chemistry in which substances do not dissolve in each other; for example, sodium chloride (salt) is not soluble in propanone (acetone). As a general rule substances can be broadly classified as polar and non-polar substances. The polar substances are found in inorganic chemistry and the non-polar substances in organic chemistry. The implication is that polar solutes like sodium chloride dissolve in polar solvents like water, and non-polar solutes like naphthalene dissolve in non-polar solvents like petroleum ether (a hydrocarbon).
4. Saturated and super-saturated solutions. A saturated solution is formed when no more solute will dissolve in a fixed volume of solvent at a constant temperature. The easiest way to obtain a saturated solution is to dissolve the solute in the solvent until excess solute is present and then to filter the solution. A supersaturated solution is one which contains more dissolved solute than is needed to form a saturated solution at the same temperature; these solutions are usually made from hydrated salts like sodium thisulphate, Na2S2O3.5H2O or sodium carbonate Na2CO3.10H2O.
5. Solubility. The solubility of a solute in a solvent is defined as the maximum number of kilograms of the solute which can be dissolved in 100 kilograms of solvent at a fixed temperature in the presence of undissolved excess solid.
6. Solubility curves. When the solubility of ionic solids in water is investigated at different temperature the solubilities are found to vary. By finding the solubilities of a solute at different temperatures the results can be displayed graphically as the solubility curves shown in Figure 43.1.
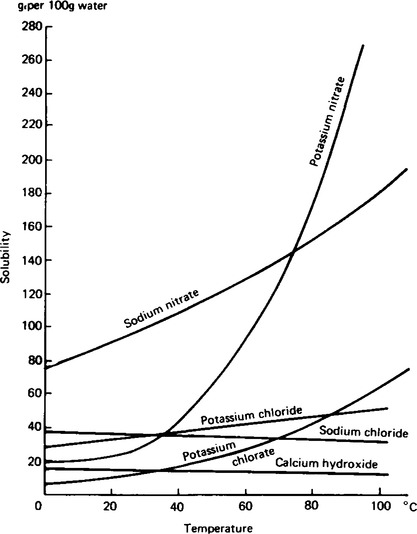
Most substances behave like potassium nitrate and sodium nitrate in that solubility usually increases with temperature.
7. A true solution is one in which the size of the particles of the solute are so small that they cannot be observed with the eye or even by using a microscope. However, as the size of the solute particles becomes larger then the solution changes from a true solution to a colloidal solution.
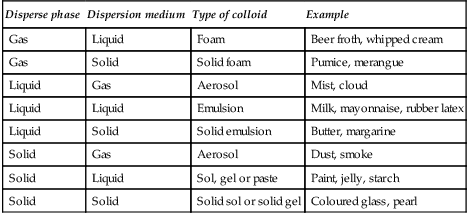
8. Colloidal solutions. A colloidal solution is formed when a disperse phase of particles which are between 1 nm and 100 nm in diameter are distributed in a dispersion medium. The disperse phase and the dispersion medium or continuous phase can be gases, liquids or solids. A colloidal solution can be composed of any combination of these phases except for a mixture of gases. A variety of colloid solutions are shown in Table 43.2.
Table 43.2
Some examples of colloidal systems
| Disperse phase | Dispersion medium | Type of colloid | Example |
| Gas | Liquid | Foam | Beer froth, whipped cream |
| Gas | Solid | Solid foam | Pumice, merangue |
| Liquid | Gas | Aerosol | Mist, cloud |
| Liquid | Liquid | Emulsion | Milk, mayonnaise, rubber latex |
| Liquid | Solid | Solid emulsion | Butter, margarine |
| Solid | Gas | Aerosol | Dust, smoke |
| Solid | Liquid | Sol, gel or paste | Paint, jelly, starch |
| Solid | Solid | Solid sol or solid gel | Coloured glass, pearl |
(i) A colloidal solution, or sol, can be classified into two main types, lyophobic and lyophilic sole.
(ii) In lyophobic sols there is little or no interaction between the disperse phase and the dispersion medium, and the particles are kept dispersed by electrical charges.
(iii) Lyophilic sols form a strong interaction between the disperse phase and the continous phase such that the disperse phase is strongly absorbed by the continuous phase, and it is this absorption which keeps the particles dispersed.
(iv) Lyophilic sols can form semi-solid masses called gels or pastes when the disperse phase is present in high concentration.
10. The properties of colloidal solutions are that they:
(i) do not separate easily on standing;
(ii) undergo electrophoresis if electrically charged (see para 11);
(iii) undergo dialysis (see para 12);
(iv) show the Tyndall effect with a beam of light (see para 13);
(v) can be coagulated or precipitated by the addition of electrolyte according to the Hardy-Schultze rule (see para 14). The effects are not identical for lyophobic sols as can be seen from Table 43.3.
Table 43.3
A comparison of lyophilic and lyopholic sols
| Lyophilic sols | Lyophobic sols |
| Stability due to adsorption of the dispersion medium | Stability due to electrical charges |
| Prepared by direct mixing and are reversible sols | Prepared by indirect methods and are irreversible sols |
| Commonly form gels | Do not usually form gels |
| Undergo electrophoresis only if the particles are charged | Undergo electrophoresis |
| Exhibit the Tyndall effect weakly | Exhibit the Tyndall effect clearly |
| Co-agulated by high concentrations of electrolyte | Co-agulated by low concentrations of electrolyte |
| Purified by dialysis | Purified by dialysis |
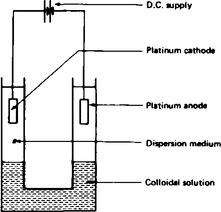
11. The effects of electrical fields oa colloids. The apparatus used to investigate the effect of an electrical field is shown in Figure 43.2. When a potential difference is set up between the cathode and the anode, the colloidal particles move towards the cathode or the anode depending upon the charge carried by the disperse phase. The movement of particles can be observed by setting up a boundary between the colloid and its dispersion medium. If the colloidal solution is coloured the effect can be more easily observed. Examples of negatively charged colloids are starch, clay and metallic particles in water. Examples of positively charged colloids are iron (III) hydroxide and aluminium hydroxide solutions. This process of the movement of charged particles is called electrophoresis.
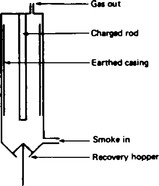
A second property of colloids in electrical fields is the electrostatic precipitation of solid particles from a smoke or mist. The passage of smoke or mist up a chimney to which a high tension d.c. supply has been fitted, can be used industrially to recover solids which otherwise would be lost to the atmosphere. A diagram showing an electrostatic precipitator is shown in Figure 43.3.
12. Dialysis of colloids. When a solvent is carefully placed on top of a true solution, the solute will diffuse up into the solvent layer until eventually the solution becomes homogeneous.
If a colloidal solution is placed in contact with its dispersion medium, the boundary between the two layers will remain definite for a much longer period of time. This is due to the much larger size of the disperse phase particles. This difference in diffusion rates can be used to purify colloidal solutions. For example, when iron (III) hydroxide is prepared from iron (III) chloride and water, the colloidal solution is contaminated by hydrochloric acid. If this mixture is placed in a cellophane tube and then immersed in running water, the hydrochloric acid which is a true solution diffuses more rapidly than the iron (III) hydroxide which is a colloidal solution. This results in the removal of the hydrochloric acid leaving a purified iron (III) hydroxide sol. This process is called dialysis.
13. The effect of a light beam on colloids When a beam of light is passed through a true solution it does not undergo any dispersion effects. However, if the same beam of light is passed through a lyophobic sol, the passage of the light is easily defined. Car headlights in fog or a cine projection through a smoky room are good examples of this effect, which is called the Tyndall effect.
The dispersion of light is caused by the difference in refractive indices between the disperse phase and the dispersion medium. Lyophilic sols tend to be more homogenous than lyophobic sols and the difference in refractive indices tends to be much smaller resulting in only slight, if any dispersion effects. Thus a beam of light can be used to identify a lyophobic sol, but not always a lyophilic sol.
14. The effect of adding electrolytes to colloids The addition of an electrolyte to a lyophobic sol results in the coagulation of the sol. The addition of a negatively charged ion causes the coagulation of a positively charged colloidal particle and vice versa. The amount of coagulation caused by a particular electrolyte is due to the valency of the coagulating ion. Thus for equal concentrations, an aluminium ion Al3+, which is trivalent will coagulate a negatively charged colloidal solution more effectively than say a sodium ion, Na+ which is monovalent. Similarly, phosphate ions PO3-4 will be more effective in coagulating a positively charged sol than a chloride ion. This is called the Hardy-Schultze rule.
Examples of the commercial use of coagulating agents are, the use of aluminium sulphate in treating dirty water, the coagulation of rubber latex using methanoic acid and the curdling of milk by 2-hydroxypropanoic acid CH3CHOHCOOH. An example of coagulation in nature is the formation of deltas in rivers when river water meets the higher Concentration of sodium chloride in sea-water. Lyophilic sols can only be precipitated or coagulated by the addition of high concentrations of electrolytes.
15. Different types of colloids are prepared in different ways, lyophobic sols by indirect methods usually involving chemical reactions and lyophilic sols by direct mixing after obtaining a disperse phase of the appropriate size. Some examples are given in Table 43.4
Table 43.4
Some methods of preparing colloidal systems
| Preparation of lyophobic sots | Preparation of lyophilic sols |
| Sulphur sol Na2S2O1(aq) + 2HCl(aq) = 2NaCl(aq) + SO2(g) + H2O(l) + S(s) | Milling Method Crush the solid into fine particles and disperse into the continuous phase |
| Iron (III) hydroxide sol FeCl1(s)+ 3H2O(l) = Fe(OH)1(s) + 3HCl (aq) | Arc Method Striking an arc between metallic electrodes immersed in a dispersion medium |
| Silver sol Reduce ammoniacal silver nitrate solution with a reducing sugar |
