The structure of materials
Publisher Summary
This chapter focuses on the structure of materials. Materials are the collective name given to substances that exist in nature in a stable state. They can be pure elements, for example, chlorine gas, bromine liquid or metallic copper solid, and pure compounds of elements. The structure of materials is an extension of the bonding or the combination of atoms into elements or compounds considers only the constituent particles. However, the structure of materials and their properties takes into account the vast number of particles that make up observable masses of material. For example, when sodium chloride is considered, only enough particles are used to define the unit cell. The structures of solid materials are all based upon particles being held in a regular three-dimensional pattern. The properties of a solid depend on the nature and intensity of the forces holding the particles in place. On the other hand, when solids undergo phase changes to become liquids and gases, their structural properties change completely and behave in the same way as materials that exist as liquids and gases at room temperature.
1. Materials is the collective name given to substances that exist in nature in a stable state. They can be pure elements, e.g. chlorine gas, bromine liquid or metallic copper solid, pure compounds of elements, e.g. hydrogen chloride gas, ethanol liquid or sodium chloride (rock salt) solid, or mixtures of different substances, e.g. brass, an alloy of metals and tungsten carbide, an interstitial compound.
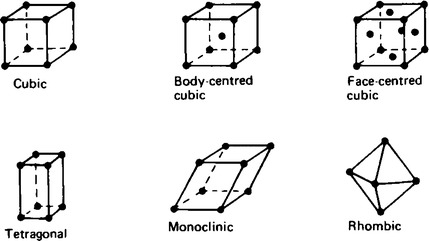
2. The structure of materials is an extension of the bonding ideas discussed in Chapter 56 and 57. The combination of atoms into elements or compounds considers only the constituent particles. However, the structure of materials and their properties takes into account the vast number of particles that make up observable masses of material. For example, when sodium chloride is considered, only enough particles are used to define the unit cell (shown in Chapter 57) but a grain of salt used for catering purposes will contain approximately 1016 particles. It is the properties of this and larger numbers of particles that are considered here.
3. There are three states of matter which material can adopt, solid liquid and gas. Of these the solid state offers a wider variety of structures than the liquid or gas states.
Ionic materials
5 When a solution of an ionic solid is allowed to evaporate very slowly, very small well defined crystals will be deposited and all of the crystals will be the same shape. Some examples are shown in Figure 65.1.
When the rate of evaporation increases, the well defined shapes disappear into clusters of crystals which grow over each other in various directions. Ionic crystals can be cleaved using a sharp cutting edge but not as easily in all directions. This is because the forces holding the solid together are not equal throughout the structure. The properties of ionic solids are summarised in Table 65.1.
Table 65.1
| Ionic solids | Covalent solids |
| 1. High melting point and high boiling point. | 1. Can be high or low melting point and boiling point. |
| 2. Soluble in water. | 2. Usually insoluble in water. |
| 3. Conduct electricity when molten or in aqueous solution. | 3. Usually non-conductors except for graphite. |
| 4. Can be cleaved along certain axes. | 4. Extremes of hardness and softness, e.g. diamond and graphite. |
Covalent solids
6. Unlike ionic solids the properties of covalently bonded solids are quite diverse. In the elemental state carbon can exist as diamond which is one of the hardest structures known, and graphite which is a very soft material sometimes used as a lubricating agent. The difference in properties is explained by their respective structure (see Chapter 56). Diamond is a network of carbon atoms all of which are held in place by four links to other carbon atoms giving a very rigid structure. In graphite the carbon atoms are only linked to three other atoms resulting in the formation of layers of atoms only weakly attracted to each other; these layers can slip over each other and relieve the friction between two metal surfaces. Other solids like sulphur, phosphorus and iodine are not extensive networks, but small molecules held in place by weak attractive van der Waal forces. These solids are brittle, and easily crushed to a fine powder.
Compounds which are covalently bonded can also exist in crystalline form, quartz being an ideal example. In quartz, silicon and oxygen are extensively covalently bonded into a diamond type network. Crystals of this type are not as easily cleaved as ionic crystals. The properties of covalent solids are summarised in Table 65.1.
Metals
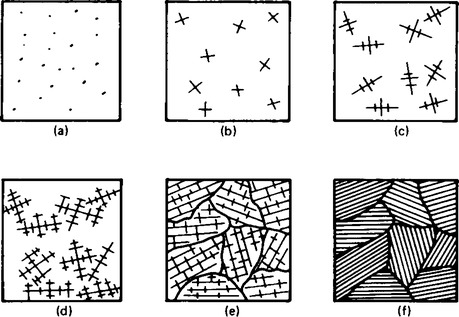
7 The close packing of metal atoms together is discussed in Chapter 56. On a much larger scale, the close-packing of atoms together as a molten metal solidifies is considered to take place over a series of steps shown in Figure 65.2. The nuclei (a), form into small crystals (b), which then form combinations of atoms called dendrites (c), which continue growing in a certain orientation (d) until they meet a set of dentrites growing in a different orientation when a boundary is formed (e) around the dentrites. This structure is called a grain structure (f). The grain structure of metals determines their properties. The size of the grains in some metals can be changed by cold-working, using such methods as rolling, extruding and pressing. In all cases the grains are elongated as shown in Figure 65.3. After such treatment the metals become harder and less ductile. Metals treated in this way can be softened by annealing, a reheating process. The internal stresses introduced by cold-working are relieved in some metals by the formation of fine grains in their new shape producing a softening of the metal. Some metals behave in this way at room temperature explaining why metals like tin and lead cannot be work hardened.

Imperfections in metal structures
8. (a) Vacant sites. If an atom is missing from the regular order of atoms, the space created is called a vacant site, shown diagrammatically in Figure 65.4. Metals’ atoms can move from one position to another, filling one site and leaving another vacant. This process is called diffusion, and is increased by a rise in temperature.

(b) Dislocations When part of a plane of atoms is missing rather than a single atom, an edge dislocation is formed as shown in Figure 65.5. The movement of these dislocations within the metal is called slip. Under stress, the dislocations tend to accumulate resulting in a deformation of the metal structure.
9. When materials are subjected to repeated stresses they may break more easily than expected due to metal fatigue, caused by the accumulation of dislocations in one particular orientation.
Alloys
10. When two or more metals are melted, mixed together and allowed to cool, the atoms of the metals can become dispersed in each other and form alloys, for example, copper and zinc in brass. In other alloys the metals crystallise out separately, for example, tin and lead in plumbers solder. The properties of an alloy are different to the metals used to make them, for example, brass is much harder than copper or zinc. Some examples of alloys are given in Table 65.2.
Table 65.2
The composition of some alloys
| Alloy | Composition |
| Plain carbon steel | Fe, 0.45%C, small amounts of Si, Mn and S. |
| Cobalt steel | Fe, 2% Co, 4% Cr, 0.9% C |
| Bronze | Cu, 0–15% Sn |
| Brass | Cu, 0–36% Zn |
| High-tensile brass | 57% Cu, 37% Zn, small amounts of A1, Mn, Fe |
| Monet, cupronickel | Ni, 30% Cu |
| Plumbers solder | 70% Pb, 30% Sn |
| Gunmetal | Cu, 5% Sn, 5% Zn |
Steel
11. Steel can be considered to be an alloy of iron and carbon, the carbon occupying certain sites in the iron metallic structure. Mild steel used in industry contains about 0.3% carbon. The carbon atoms reduce the amount of movement which can occur in the metallic structure, and increases the strength of the structure. High carbon steel contains up to 1% carbon and are very hard steels used for cutting tools. Other types of steel contain other elements, for example, stainless steel contains up to 18% chromium as well as traces of carbon. The heat treatment of steel has the same effect as in iron, rapid or gradual cooling giving different sizes of grains and hence different properties.
Interstitial compounds
12. The presence of carbon in steel, changes the properties considerably. Other small atoms like hydrogen and nitrogen can also be enclosed within the structure of the metal, and cause similar effects. For example, nitrogen and carbon in metals leads to the formation of hydrides, nitrides and carbides. The nitrides and carbides are very hard materials; for example, tungsten carbide is used to drill holes in masonry and rocks. The presence of these elements in metals prevents the movement of dislocation through a structure causing more rigid structures.
Polymer structures
13. Polymers are very large molecules formed by the joining together of smaller molecules called monomers, by the process of polymerisation. There is a large variety of polymers showing many different properties. They can be broadly classified in terms of their physical properties as, thermoplastics, thermosetting plastics, fibre polymers and rubbers. The structure of a polymer depends upon its method of preparation and the monomer units it contains. For example, the long chain molecules which constitute thermoplastics are held together in the solid state by van der Waal forces, whereas in thermosetting plastics extensive cross-linking occurs within the polymer structure.
Thermoplastics
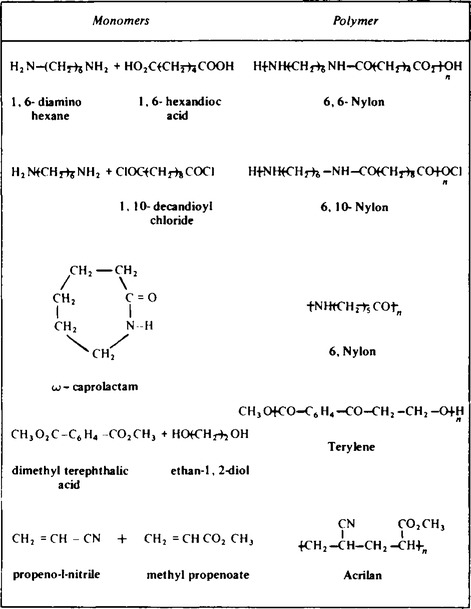
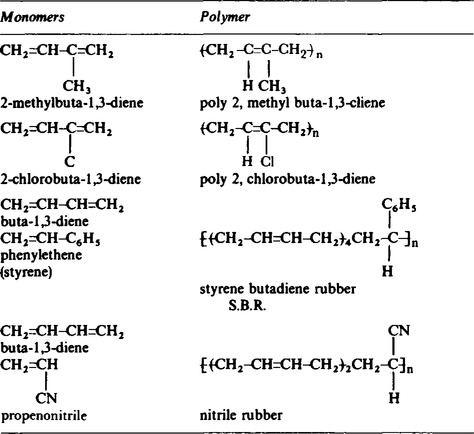
14. These are usually made from monomer units which contain a carbon to carbon bond. Some examples are given in Table 65.3. An example of this type of reaction is the formation of polyvinyl chloride P.V.C. from chloroethane,
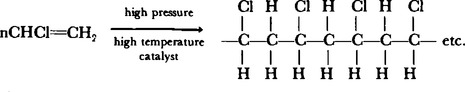
These polymers can be changed back to the monomer by distillation though it is not a useful process at this time. The long chain molecules are packed together in a somewhat ordered fashion, giving a certain degree of tensile strength. The materials can be hot moulded into shape by melting the plastics and blowing them into the mouldings. When cooled, this shape will be maintained unless the plastic is heated in which case it becomes deformed. The forces holding the chains of molecules together are not rigid and can be broken fairly easily.
Thermosetting plastics
15. These are all co-polymers and form large extensively cross-linked molecules as shown in Table 65.4. These plastics as the name suggests form a rigid solid as the reaction occurs. This means the plastics must either be moulded during reaction, or by machining after reaction. The plastics are brittle and do not melt but decompose. They are used for making heat resistant materials like ashtrays and electrical fittings.
Fibre polymers
16. These polymers can be co-polymers or made from a single monomer, some examples of which are shown in Table 65.5. They are similar to thermoplastics in that they can be extruded under heating, but are much more stronger than thermoplastics. After extrusion as fine fibres the polymers are twisted into much thicker fibres and then formed into a variety of materials ranging from monofilament fishing line to mooring ropes used in the shipping industry.
Rubbers
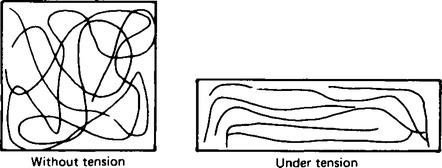
17. Natural rubber is obtained from the sap of the rubber tree. After suitable treatment it is obtained as a clear flexible solid. In this, and in the synthetic rubbers like styrene-butadiene rubber as shown in Table 65.6., the structure of the material is such that under tension thesolid elongates, but after the tension is released the original shape is reformed. This is due to the retangling of the polymer chains that are partially untangled under tension as shown in Figure 65.6. However, if the elastic limit is exceeded the original shape will not be reformed, but remains deformed.
The process of vulcanisation which is the addition of sulphur to the rubber causes cross-linking to occur between the polymer chains. The greater the amount of vulcanisation the more rigid the rubber becomes.
18. The structure of plastics and polymers can be changed dramatically by the introduction of additives. The major additives are fillers which increase bulk and rigidity, plasticiser which increase the flexibility, dyes which control colour and stabilisers which increase the useful life in the atmosphere or in water. The use of different combinations of additives can change the structure to the extent that polyvinychloride can be used as a flexible clothing material or the rigid pipes used for transportation of materials below the earth’s surface.