Rates of chemical reaction
Publisher Summary
This chapter discusses that the rate of a chemical reaction can be obtained by either measuring the amount of products formed or by measuring the amount of reagents used up in a given time. Chemical reactions can be considered as a rearrangement of elements, or groups of elements, into new patterns. Each particular chemical reaction takes place at an individual rate which can be very slow or very rapid. Because of this large variation in reaction time, it is apparent that under a set of conditions one reaction might not take place while another takes place with ease. Chemical reactions do not all take place in the same way. Some chemical reactions take place by a one step mechanism while others take place as a result of a number of steps each equivalent to the formation of an intermediate product before further reaction occurs. When a multi-stage reaction occurs, the rate of the reaction is taken to be that of the slowest step in the reaction mechanism. The rate of a reaction has been found experimentally to depend upon certain factors such as temperature (all reactions); concentration (nongaseous systems); pressure (gaseous systems); catalysts (all reactions); and particle size.
1. The rate of a chemical reaction can be obtained by either measuring the amount of products formed or by measuring the amount of reagents used up in a given time.
2. Chemical reactions can be considered as a rearrangement of elements, or groups of elements, into new patterns. Each particular chemical reaction takes place at an individual rate which can be very slow or very rapid. Because of this large variation in reaction time, it is apparent that under a set of conditions one reaction might not take place whilst another does take place with ease.
3. Since reactions depend upon the breaking and forming of chemical bonds, energy must be of prime importance in considering rates of reaction. However, since reactions can take place in many different ways no single theory of reactions has been established.
4. Chemical reactions do not all take place in the same way. Some chemical reactions take place by a one step mechanism whilst others take place as a result of a number of steps each equivalent to the formation of an intermediate product before further reaction occurs. When a multi-stage reaction occurs, the rate of the reaction is taken to be that of the slowest step in the reaction mechanism.
5. The rate of a reaction has been found experimentally to depend upon certain factors. These factors are
(i) temperature (all reactions);
(ii) concentration (non-gaseous systems);
(iii) pressure (gaseous systems);
6. Methods used to compare rates of chemical reactions include the measurement of volumes of gas evolved, the titration of acids and bases or redox systems and colorimetric measurements.
(i) To investigate the effect of temperature, different thermostated heating baths must be used, all other factors being kept constant.
(ii) To investigate the effect of concentration, different concentrations of one of the components is used, everything else being kept constant.
(iii) The effect of pressure is more difficult to monitor in a simple experiment; the variation of pressure must be the only variable in this investigation.
(iv) To investigate the effect of a catalyst, the rate of reaction can be compared with and without a catalyst.
(v) The effect of particle size can be investigated by using a single large lump of substance and comparing its rate to that of the same mass of substance crushed into smaller pieces.
7. The fact that chemical reactions take place over a wide range of rates (including no reaction to spontaneous explosive reactions), has been investigated by the application of the kinetic theory of matter.
The collision theory of chemical reactions
8. Consider bimolecular reactions of the type, A + B → Products for a reaction to occur, existing chemical bonds must be broken, and new bonds must be formed. In order to explain qualitatively why reaction rates can be increased by changing various factors, the collision theory has been formulated. It has been suggested that the reacting molecules must collide together, which is a statement of the kinetic theory of matter. Thus, the rate of a reaction is dependent on collisions occurring between molecules.
In the gas phase many reactions do not occur until the temperature of the reactants is high enough. The collision theory suggests that since the increase in temperature increases the motion of the molecules, more collisions will occur between molecules A and B, and hence the reaction rate increases. If a reaction is at a high enough temperature to occur, an increase in the pressure of the gas mixture also increases the rate of the reaction. This can be achieved either by reducing the volume available to the mixture or by increasing the number of molecules. Whichever process is used, the number of collisions must be increased.
For reactions in the liquid phase or in solution, reaction rates can be increased by increasing the temperature or the concentration of the reactants, both of these factors increasing the number of collisions which take place in the reaction.
For a reaction in which a solid is involved, the rate of reaction can be increased by increasing the state of division of the solid. This increases the surface area at which collisions can occur and hence the collision theory qualitatively explains this by an increased number of collisions.
9. A further point of interest is that it can be shown that for a particular number of molecules in a given volume and at a constant temperature, the number of collisions which occur, does not reflect the rate at which reaction takes place.
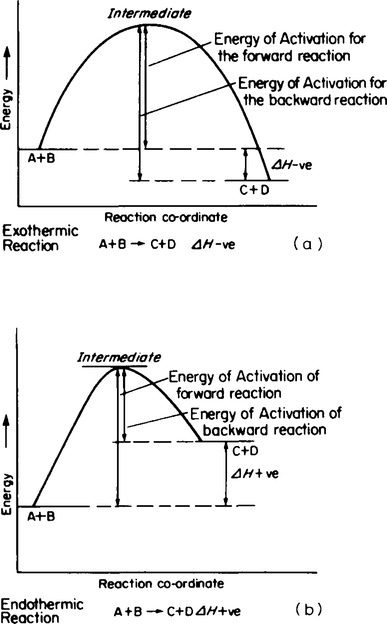
10. It was suggested by Arrhenius that reaction only takes place between activated molecules which have an activation energy in excess of a particular value. This theory implied that, unless the interacting molecules collide with suffcient energy, reaction will not take place. Alternatively, if they collide with an energy much greater than the energy required an extremely vigorous reaction would take place. This prevention of reactions occurring led to the idea of an energy barrier which has to be overcome before reactions can occur. This allows reaction profile diagrams to be drawn for reactions as shown in Figure 60.1.
11. The above theory was placed on a quantitative basis by a consideration of the number of molecules N of a total number of molecules No having the requisite amount of energy E (the Activation energy) for a reaction to occur. Maxwell and Boltzmann showed that the relationship between these values approximated to a normal distribution which can be expressed in a simplified equation as:
where R is the gas constant and T is the absolute temperature.
12. It has already been stated that the rate of a reaction is dependent upon the number of effective collisions which occur in the reaction. Hence, the rate of a reaction is proportional to the ratio N/No but the ratio N/No is itself proportional to e−E/RT. Thus, the rate of reaction, k, can be related to the Activation energy E by the equation
k= Ae−E/RT where A is called the Arrhenius constant.
Taking logarithms of this equation gives:
For two different temperatures T1 and T2 with corresponding rate constants k1 and k2 the relationship between these values can be expressed in the form
This means that if T1, E, R and k1 are known, the rate of the reaction at any other temperature T2 can be found. For example, at 730 K, the rate constant k1 for the reaction
is 5 × 10−1 mol−1 s−1 the energy of activation E is 105 kJ mol−1 and gas constant R=0.00832 kJ mol−1 K−1. The rate constant k2 at 780 K is found by using equation (1), i.e.
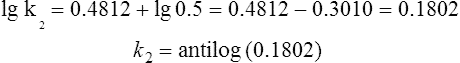
Thus the rate constant at 780 K is 1.514 mol−1 s−1. Hence for an increase of 50 K the rate constant is increased threefold.
The role of catalysts in chemical reactions
13. catalyst can be defined as a substance which will alter the rate of a chemical reaction, but remaining chemically unchanged at the end of the reaction. Since the rate of a chemical reaction is dependent upon the energy of activation an alternative definition could be that a catalyst is a substance which changes the energy of activation of a reaction, itself remaining unchanged at the end of the reaction. Some energy profile diagrams showing positive and negative catalysts are shown in Figure 60.2. Positive catalysts are those which speed up reactions to a suitable rate whereas negative catalysts are used to slow down reactions which under normal conditions would be explosive or uncontrollable.

