Ideal gas laws
Publisher Summary
The relationship that exists among pressure, volume, and temperature of a gas are given in a set of laws called the gas laws. An ideal gas is one that completely obeys the gas laws. In practice, no gas is an ideal gas, although air is very close to being one. The kinetic theory of gases suggests that gases are composed of particles in motion. The continual bombardment of any surface by the gas causes a pressure to be exerted; the greater the densityof a gas, the more frequent the number of collisions between molecules and the surface, and the greater the pressure exerted. Therefore, the pressure increases either when the volume of a certain mass of gas is reduced, or when more gas is pumped into a vessel. When the temperature of a gas is increased, the speed of themolecules increases, causing an increase in both the number and the momentum imparted by each collision. This accounts for the increase in pressure of a gas with increase in temperature.
1. The relationship which exists between pressure, volume and temperature of a gas are given in a set of laws called the gas laws.
‘the volume V of a fixed mass of gas is inversely proportional to its absolute pressure p at constant temperature’
i.e ![]() , at constant temperature,
, at constant temperature,
p = absolute pressure in pascals (Pa),
(ii) Changes which occur at constant temperature are called isothermal changes.
(iii) When a fixed mass of gas at constant temperature changes from pressure p1 and volume V1 to pressure p2 and volume V2 then:
‘for a given mass of gas at constant pressure, the volume V is directly proportional to its thermodynamic temperature T’
i.e. V ∝ T or V = kT or ![]() = k, at constant pressure,
= k, at constant pressure,
where T = thermodynamic temperature in kelvin (K).
(ii) A process which takes place at constant pressure is called an isobaric process.
(iii) The relationship between the Celsius scale of temperature and the thermodynamic or absolute scale is given by:
i.e. K = °C + 273 or °C = K − 273
(iv) If a given mass of gas at constant pressure occupies a volume V1 at a temperature T1 and a volume V2 at temperature T2, then
For example, a gas occupies a volume of 1.2 litres at 20°C. If the pressure is kept constant, the volume it occupies at 130°C is determined from
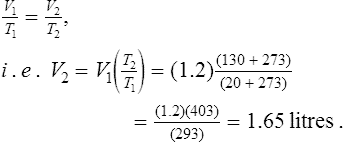
‘the pressure p of a fixed mass of gas is directly proportional to its thermodynamic temperature T at constant volume’
(ii) When a fixed mass of gas at constant volume changes from pressure p1 and temperature T1, to pressure p2 and temperature T2 then:
(i) Dalton’s law of partial pressure states:
‘the total pressure of a mixture of gases occupying a given volume is equal to the sum of the pressures of each gas, considered separately, at constant temperature’.
(ii) The pressure of each constituent gas when occupying a fixed volume alone is known as the partial pressure of that gas.
6. An ideal gas is one which completely obeys the gas laws given in paras 2 to 5. In practice no gas is an ideal gas, although air is very close to being one. For calculation purposes the difference between an ideal and an actual gas is very small.
(i) Frequently, when a gas is undergoing some change, the pressure, temperature and volume all vary simultaneously. Provided there is no change in the mass of a gas, the above gas laws can be combined giving:
(ii) For an ideal gas constant k = mR, where m is the mass of the gas in kg, and R is the characteristic gas constant,
This is called the characteristic gas equation. In this equation, p = absolute pressure in pascals, V = volume in m3, m = mass in kg, R = characteristic gas constant in J/(kg K) and T = thermodynamic temperature in kelvin.
(iii) Some typical values of the characteristic gas constant R include: air, 287 J/(kg K), hydrogen 4160 J/(kg K), oxygen 260 J/(kg K) and carbon dioxide 184 J(kg K). For example, some air at a temperature of 40°C and pressure 4 bar occupies a volume of 0.05 m3. The mass of air is determined from
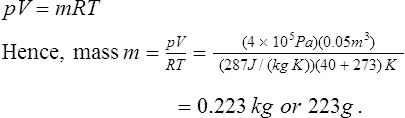
8. Standard temperature and pressure (i.e. STP) refers to a temperature of 0°C, i.e. 273 K, and normal atmospheric pressure of 101.325 kPa.
Kinetic theory of gases
9. The kinetic theory of gases suggests that gases are composed of particles in motion. The continual bombardment of any surface by the gas causes a pressure to be exerted; the greater the density of a gas, the more frequent the number of collisions between molecules and the surface and the greater the pressure exerted. Hence the pressure increases either when the volume of a certain mass of gas is reduced, or when more gas is pumped into a vessel. When the temperature of a gas is increased, the speed of the molecules increases, causing an increase in both the number and the momentum imparted by each collision. This accounts for the increase in pressure of a gas with increase in temperature.
Maxwell (in 1860) explained some of the properties of a gas by assuming that the molecules of a gas make elastic collisions, spend negligible time actually in collision, and themselves occupy a negligible part of the volume of the gas. Also, the attractive forces between molecules are assumed negligible.
10. It may be shown that for a gas occupying a volume V at pressure p and containing n molecules each of mass m moving at an average velocity of c,
Also, the kinetic energy of the molecules of a gas is proportional to its thermodynamic temperature.
11. When a liquid evaporates molecules with sufficient kinetic energy escape from the liquid’s surface. The higher the temperature of the liquid the greater the average kinetic energy of the molecules and the greater the number of molecules which are able to escape. Since it is the molecules with the highest kinetic energy which escape the average kinetic energy of the remaining molecules decreases and thus the liquid cools.
12. If a liquid evaporates a vapour is formed. When a vapour exists in the presence of its own liquid a saturated vapour is formed. If all the liquid evaporates an unsaturated vapour i is produced. The higher the temperature the greater the number of molecules which escape to form the vapour. These molecules bombard the walls of the container and thus exert a pressure.
The saturated vapour pressure depends only on the temperature of the vapour. The saturated vapour pressure of water at various temperatures is shown in Table 46.1.
Table 46.1
| Temperature (°C) | Saturated vapour pressure of water (103 Pa) |
| 0 | 0.61 |
| 10 | 1.23 |
| 20 | 2.33 |
| 30 | 4.23 |
| 40 | 7.35 |
| 50 | 12.3 |
| 60 | 19.9 |
| 70 | 31.2 |
| 80 | 47.4 |
| 90 | 70.2 |
| 100 | 101 |
| 150 | 476 |
| 200 | 1550 |
A liquid boils at a temperature when its saturated vapour pressure is equal to the atmospheric pressure. Thus water will boil at a temperature greater than 100°C if the atmospheric pressure is increased. This is the principle of the pressure cooker.
13. A saturated vapour does not obey the gas laws since its pressure depends only on temperature. An unsaturated vapour will obey the gas laws fairly closely as long as it remains unsaturated. If an unsaturated vapour at a particular temperature is decreased in volume its pressure will rise in accordance with Boyle’s law until it reaches the saturated vapour pressure at that particular temperature (see Figure 46.1). When the vapour pressure at 40°C reaches 7.35 × 103 Pa the vapour becomes saturated as it starts to liquify.

