Energy of chemical reactions
Publisher Summary
This chapter focuses on the energy of chemical reactions. The energy of atoms and molecules in a chemical system is made up of kinetic energy and potential energy. The total energy of a chemical system is called the internal energy and is given the symbol U. The total amount of internal energy associated with a system is difficult to measure absolutely, but changes in energy can readily be determined. The change in internal energy is signified as ΔU. The unit of heat energy is the joule J, and the molar heat capacity of a system is measured in joules per mole Kelvin. A consideration of the internal energy of a chemical system involving several polyatomic molecules is difficult. To obtain information about energy changes that occur, the measurements are made at constant pressure and hence are called the enthalpy changes, ΔH. When a chemical reaction releases heat energy to its surroundings, it is called an exothermic reaction whereas when a reaction absorbs heat from its surroundings it is called an endothermic reaction. The law of Hess states that the total change in enthalpy in a chemical reaction is independent of the number of stages used to complete the reaction.
1. It has been previously stated in Chapter 37, para 4 that energy is the capacity of a system to do work. The energy of atoms and molecules in a chemical system is made up of kinetic energy and potential energy. The kinetic energy is due to:
(a) transitional energy, i.e. the molecules in motion; for example, molecules in the liquid and gas phase,
(b) rotational energy, i.e. the rotation of the molecules and
(c) vibrational energy, i.e. the vibration of atoms in a molecule about a specific position. The potential energy is due to the repulsive and attractive forces between the particles in a molecule.
2. The total energy of a chemical system is called the internal energy and is given the symbol U. The total amount of internal energy associated with a system is difficult to measure absolutely, but changes in energy can readily be determined. The change in internal energy is signified as ΔU.
3. The unit of heat energy is the joule J, and the molar heat capacity of a system is measured in joules per mole Kelvin or J mol−1 K−1
The internal energy of a monatomic gas
4. A consideration of the noble gas which exists in the monatomic state shows that there is no internal energy due to potential energy. The only energy is kinetic energy. The kinetic theory of matter (Chapter 58) derives the fundamental equation:
for 1 mole of gas, and the ideal gas law for one mole of gas is:
The total internal energy is U, and since the average kinetic energy of 1 mole of gas is 1/2Nmu−2 then
Differentiating with respect to T gives:
The expression dU/dT is the rate of change of the internal energy with temperature, which is the energy change associated with 1 degree Kelvin. This is defined as the molar heat capacity of the gas at constant volume and symbolised Cv. Hence
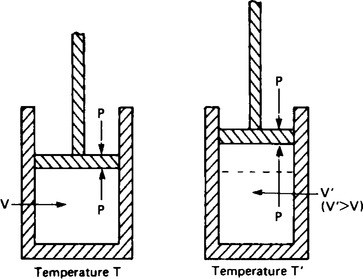
5. Let 1 mole of a monatomic gas be contained in a cylinder fitted with a weightless friction-free piston as shown in Figure 61.1, and let the pressure due to the gas above and below the piston be P and the volume of the gas be V. If the cylinder is heated, the kinetic energy of the gas increases and causes an increase in temperature. This increases the force acting on the piston and the piston will move to accommodate the increased volume of the gas. The heat energy given to the cylinder increases the internal energy of the gas and in addition does work in moving the piston.
This can be represented by the equation
the first law of thermodynamics, where q is the heat energy supplied, ΔU is the change in internal energy and w is the work done by the system. This means that the value of Cp, the molar heat capacity at constant pressure, is greater than Cv according to the equation:
where the work done=pressure × increase in volume.
For a pressure P the work done in changing the volume from V to V’ is
The work done can be expressed as
The temperature change for the molar heat capacity is 1 K hence
This means that for a monatomic gas
Cv has been shown to be 12.465 Jmol−1 K−1 and since R=8.314 Jmol−1 K−1 then Cp= 20.779 Jmol−1 K−1. For a diatomic gas it can be shown that Cv= 20.8 and Cp= 29.1 Jmol−1 K−1 and for a triatomic gas Cv= 25.0 and cp= 33.3 Jmol−1 K−1.
Hence at constant pressure, the heat energy absorbed is a combination of the internal energy of the system and the work done by the system. This is represented by the equation:
Exothermic and endothermic reactions
7. When a chemical reaction releases heat energy to its surroundings it is called an exothermic reaction and is represented by the equation:
When a reaction absorbs heat from its surroundings it is called an endothermic reaction and is represented by the equation:
Some definitions of standard ethalpy changes
9. The standard enthalpy of combustion ΔH![]() c is the enthalpy change when one mole of a substance (the relative molecular mass, in grams) is completely burned in oxygen, under standard conditions. For example:
c is the enthalpy change when one mole of a substance (the relative molecular mass, in grams) is completely burned in oxygen, under standard conditions. For example:
The standard enthalpy of formation, ΔH![]() f the enthalpy change when one mole of a substance is prepared from its elements, under standard conditions. For example
f the enthalpy change when one mole of a substance is prepared from its elements, under standard conditions. For example
The standard enthalpy of solution, ΔH![]() s is the enthalpy change when one mole of a substance is dissolved in a solvent to give a solution of defined concentration, under standard conditions. For example,
s is the enthalpy change when one mole of a substance is dissolved in a solvent to give a solution of defined concentration, under standard conditions. For example,
Since the most common solvent used in chemistry is water, the standard enthalpy of hydration ΔH![]() h is of importance. The standard enthalpy of neutralisation is the enthalpy change when one mole of aqueous hydrogen ions are neutralised by a base in dilute solution. For strong acids and strong bases, this value is – 57.33 kJ. This is because, independent of the strong acid or base, the neutralisationreaction is:
h is of importance. The standard enthalpy of neutralisation is the enthalpy change when one mole of aqueous hydrogen ions are neutralised by a base in dilute solution. For strong acids and strong bases, this value is – 57.33 kJ. This is because, independent of the strong acid or base, the neutralisationreaction is:
(For weak acids and bases, the enthalpy of neutralisation is lower because some of the heat energy produced is required by the reaction to complete the ionisation of the acids and bases).
The standard ehtalpy of atomisation is the enthalpy change which occurs when one mole of an element in its standard state at 298 K and 101.325 kPa is converted into free atoms.
The standard enthalpy of ionisation is the enthalpy change which occurs when one mole of gaseous atoms are converted into one mole of gaseous ions accompanied by a loss of one mole of electrons, measured under standard conditions. It should be noted that each element has a different number of ionisation energies dependent upon the number of electrons in the element. Usually the formation of stable metallic ions are associated with a loss of 1, 2, 3 or 4 electrons.
The standard enthalpy of lattice energy is the enthalpy change which occurs when one mole of an ionic solid is formed from its constituent gaseous ions measured under standard conditions.
The standard enthalpy of electron affinity is the enthalpy change which occurs when one mole of gaseous atoms are converted into one mole of gaseous negative ions by accepting one mole of electrons, measured under standard conditions. It should be noted that some non-metals can have more than one electron added: for example, oxygen has two electron affinity enthalpy values.
For covalent molecules the energy required to separate one mole of covalent bonds between two atoms in a diatomic molecule is called the bond dissociation enthalpy, or bond dissociation energy. Some typical examples of values are given in Table 61.1, where B.D.x−y symbolises those terms.
Table 61.1
| Molecule X − Y | Enthalpy B.D.x−y kJ mol−1 |
| H–H | 436 |
| F–F | 269.9 |
| Cl–Cl | 242.7 |
| Br–Br | 192.9 |
| I–I | 151 |
| H–F | 621.3 |
| H–Cl | 431.8 |
| H–Br | 366.1 |
| H–I | 298.7 |
In covalent molecules containing more than two atoms the bond dissociation energies are found for all of the bonds and an average value is derived. For example, in methane, CH4 there are four carbon to hydrogen bonds. The enthalpy required to break the four bonds is 1666 kJmol−1, and the average value is thus 416.5 kJmol−1.
The Born-Haber cycle for ionic solids
11. The Born-Haber cycle is an application of Hess’s law used to relate together all of the enthalpy changes involved in the formation of an ionic solid. An example of such a Born-Haber cycle is shown in Figure 61.2 for calcium sulphide. In this diagram the combined 1st and 2nd electron affinities have been omitted. To find the value, Hess’s law is applied to the system and stated as:

Substituting the known values of enthalpy changes into the equation gives:
which gives a value for the enthalpy of electron affinity of
12. For covalently bonded molecules similar enthalpy cycles as above, can be constructed to obtain information about covalent reactions. The enthalpy cycle shown in Figure 61.3 shows the interrelationships between methane and its elements. For example, the equation for the formation of methane can be written:

where by definition, ΔH1 is the enthalpy of formation of methane. Before covalent bonds are formed by atoms of carbon and hydrogen the carbon must be atomised and the hydrogen molecules dissociated into atoms. The enthalpies involved are ΔH2 and ΔH3 both of which are endothermic. Hence
ΔH2= the enthalpy of atomisation of carbon = ΔH![]() a carbon
a carbon
ΔH3 = 2 × the bond dissociation enthalpy of hydrogen = 2 x B.D.H - H
The next step involves the formation of four carbon to hydrogen single covalent bonds. Bond formation is an exothermic process and ΔH4 in the equation:
is 4 × the average bond dissociation energy of a C—H bond,
By applying Hess’s law of constant heat summation:
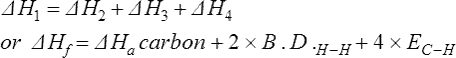
Hence by knowing the required enthalpy values, the enthalpy of formation can be calculated.
13. Another useful application of Hess’s law is the determination of the enthalpy of covalent reactions. When any reaction takes place chemical bonds must be first broken and then new bonds formed. The bond breaking is an endothermic process and bond forming an exothermic process. By considering the energy associated with covalent bonds in all of the molecules, the difference between reactants and products will be the enthalpy of the reaction. For example, the hydrogenation of ethene takes place according to the equation:

The mean bond energies of the bond to be broken and formed can be presented in tabular form as follows:
| Bonds broken | Total energy |
| 4 × C – H | 4 × 413= + 1652 |
| 1 × C=C | +598 |
| 1 × H – H | +436 |
| +2686 |
Measurement of enthalpy changes
14. The enthalpy changes which are measured are determined using calorimetry. A calorimeter is a container, which is insulated against heat loss, and contains a liquid of known specific heat capacity, c (usually water) in which the chemical reaction takes place. The rise in temperature of a known mass of liquid, m kg, from t°1 C to t°2 C, is associated with an energy change ΔH given by the expression:
(See Chapter 40, para 4.)
A simple calorimeter
15. The simple calorimeter shown in Figure 61.4, shows the precautions which are taken against heat loss. In addition to heating the water through the measured temperature range, the whole calorimeter experiences the same temperature rise using heat energy from the chemical reaction. The total energy from the reaction is a combination of these two quantities. The heat energy required to raise the temperature of the calorimeter through 1°C is called the heat capacity of the calorimeter. The heat capacity is calculated by placing a known mass of water m kg, into a calorimeter containing all of the apparatus required for a calorimetery experiment (see Figure 61.4).
An electrical heating element is then placed into the water and a steady current, I amperes, flows for a time t seconds from a supply voltage, V volts. The rise in temperature, Δθ K of the water is noted. By assuming that all of the electrical energy is converted into heat energy, and ignoring any heat lost from the outer container the electrical energy supplied is given by:
This energy heats the water and the calorimeter. The heat energy used in producing a temperature rise of Δθ K on m kg of water is given by:
where c=4.18 kJ kg−1 K−1, the specific heat capacity of water. The difference in the two values obtained from equations (1) and (2) is the heat energy required to raise the temperature of the calorimeter through Δθ K. The heat capacity of the calorimeter for a charge in temperature of Δθ K is given by the equation (1) minus equation (2), that is
The heat capacity for a rise in temperature Δθ is given by:
Heat capacity of the calorimeter
For example, when 0.6 kg of water in a calorimeter of heat capacity 0.15 kJK−1 increases in temperature from 291 K to 311 K the enthalpy change for the water, ΔH1, is given by the expression
where c for water is 4.18 kJ kg−1 K−1.
Substituting in the values for m, c, t2 and t1 gives:
The enthalpy change for the calorimeter ΔH2 is given by
ΔH2 = heat capacity of the calorimeter × (t2 − t1), that is
The total enthalpy change ΔH is given by:
By finding the enthalpy change for a known number of moles of reactants, the molar enthalpy change for the reaction can be calculated.
The bomb calorimeter
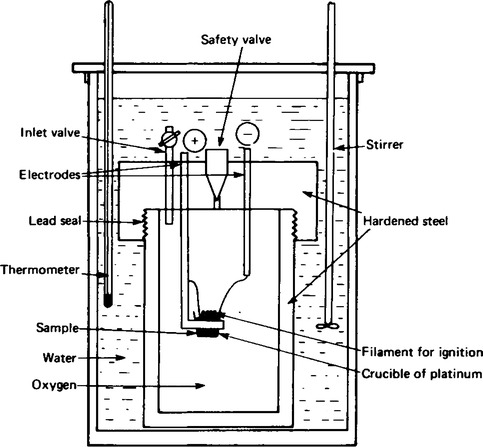
16. When the reaction taking place produces a large change in volume the construction of the calorimeter must be robust. The bomb calorimeter shown in Figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are reactions causing a large change in volume of this type. The measurements are made in the same way as in the simple calorimeter.
17. A knowledge of the enthalpy changes of chemical reactions is very useful in giving an indication of how much heat energy is available from a chemical reaction.