Chemical bonding of the elements
Publisher Summary
This chapter focuses on the chemical bonding of the elements. Chemical bonding is the technique through which the atoms of the elements are held together in molecules. The type of bonding that binds the atoms of the metallic elements together is called metallic bonding. X-ray crystallographic studies have shown that the metal atoms are closely packed together in such a way that they occupy a minimum volume, and hence a minimum potential energy exists among atoms. An ordered arrangement of spheres can be used to represent the types of bonding that occur. Some of the physical properties of most metals are that they are malleable, ductile, good conductors of heat and electricity and in addition the majority of the metals are dense and have high melting points and boiling points. The nonmetallic elements in their pure states are held together by a different type of bonding called covalent bonding. This involves the snaring of two electrons between two different atoms that holds the electrons in a distinct position relative to each other. Each element can share as many electrons as are necessary to achieve a stable electronic configuration. These stable electronic configurations are those of the inert or noble gases, neon, argon, krypton, or helium. The number of bonds formed is thus dependent on the electronic configuration of the particular element.
1. When the elements are in their pure states they can be broadly divided into metals and non-metals by consideration of their physical properties. The way in which the atoms of the elements are held together in molecules is called chemical bonding.
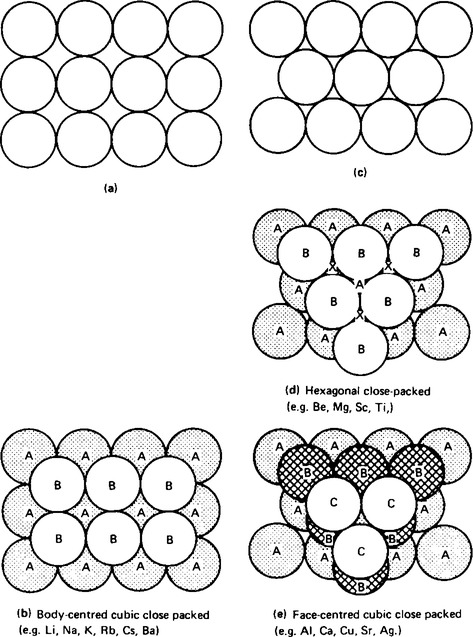
(i) The type of bonding which binds the atoms of the metallic elements together is called metallic bonding. X-ray crystallographic studies have shown that the metal atoms are closely packed together in such a way that they occupy a minimum volume, and hence a minimum potential energy exists between atoms. An ordered arrangement of spheres can be used to represent the types of bonding that occur, and the three possible structures are shown in Figure 56.1.
(ii) When the layers of atoms are arranged as shown in Figure 56.1(a), there is only one way of obtaining a minimum volume, which is shown in Figure 56.1(b). This type of arrangement is termed cubic close-packing, and the structure is called a body-centred cubic structure, in which any single atom is touching twelve other atoms. For this reason the atom is said to have a co-ordination number of twelve.
(iii) When the layers of atoms are arranged as shown in Figure 56.1(c) there are two ways in which a minimum volume can be achieved. These are shown in Figure 56.1(d) and (1(e). The structure in Figure 56.1(d) shows the first two layers of a repeating structure, i.e. AB AB AB. In this structure gaps can be seen when looking on to the arrangement of atoms indicated by the letter X in the diagram. This is called a hexagonal close-packed structure, in which each atom touches twelve others, i.e. it has a co-ordination number of twelve.
(iv) The other possible arrangement of atoms is shown in Figure 56.1(e). In this structure the gaps present in Figure 56.1(d) have been filled by placing the third layer of atoms over the gaps designated by X in that structure. This means that the repeat order of the structure is ABC ABC ABC. This structure is called a face-centred cubic structure with a co-ordination number of twelve.
3. The amount of vacant space in the three different structures is 32% in Figure 56.1(b) and 26% in Figure 56.1(d) and (e). Examples of metals having the different types of bonding are also given in Figure 56.1.
4. Some of the physical properties of most metals are that they are malleable, ductile, good conductors of heat and electricity and in addition the majority of the metals are dense and have high melting points and boiling points. These properties can be explained in terms of metallic bonding theory in the following ways.
Since the theory of metallic bonding states that the atoms of the metals are not directly bonded together, but close-packed together, it follows that the atoms within the solid can be redistributed by externally applied mechanical forces. For example, continuous hammering of a piece of metal causing it to change it’s shape is a process which is termed malleability. Similarly, metals can be drawn into a wire by the application of a tensile force to the ends of a metal rod, this being called ductility.
Good electrical conductivity is explained by the presence of non-bonded electrons in the close-packed structures. Since the electrons within the structure are essentially free electrons, the passage of a stream of electrons takes place readily.
Good thermal conductivity is explained by the ready acceptance and transfer of heat energy from one atom to another when close-packed. High density in the metals is explained by the close-packing together of the atoms in either the hexagonal, or cubic close-packed structures. The high melting points and boiling points are explained by the amount of energy required to separate the atoms from each other, and due to the close packing, a large amount of energy is required.
Hence all of the properties can be explained in terms of the close-packed non-directionally bonded theory.
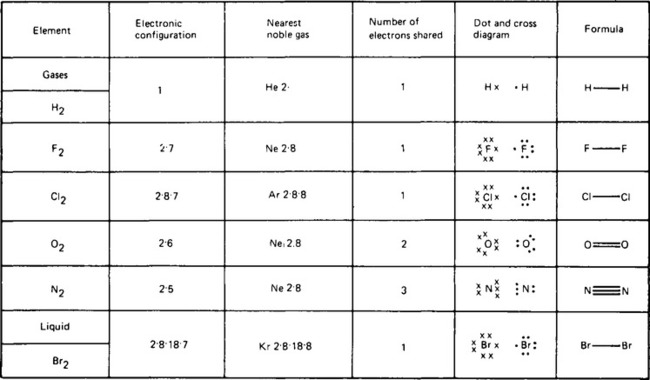
5. The non-metallic elements in their pure states are held together by a different type of bonding called covalent bonding. This involves the sharing of two electrons between two different atoms which holds the electrons in a distinct position relative to each other. Each element can share as many electrons as are necessary to achieve a stable electronic configuration. These stable electronic configurations are those of the inert or noble gases, neon, argon, krypton or helium. The number of bonds formed is thus dependent on the electronic configuration of the particular element and some examples are shown for simple gaseous elements in Figure 56.2. The number of bonding electrons surrounding each atom are shown by crosses (X) or dots (•), each pair of shared electrons forming a covalent bond. In this way, one (H—H), two (O=O) or three (N≡N) covalent bonds can be formed between two atoms of the elements.
6. One of the non-metals, bromine, is a liquid at room temperature. As can be seen from Figure 56.2, each bromine molecule contains two bromine atoms joined by one covalent bond. The reason why bromine is a liquid at this temperature rather than a gas (like chlorine) can only be explained by the presence in the structure of liquid bromine of some forces of attraction between the molecules. These forces of attraction are called van der Waal forces, or intermolecular forces.
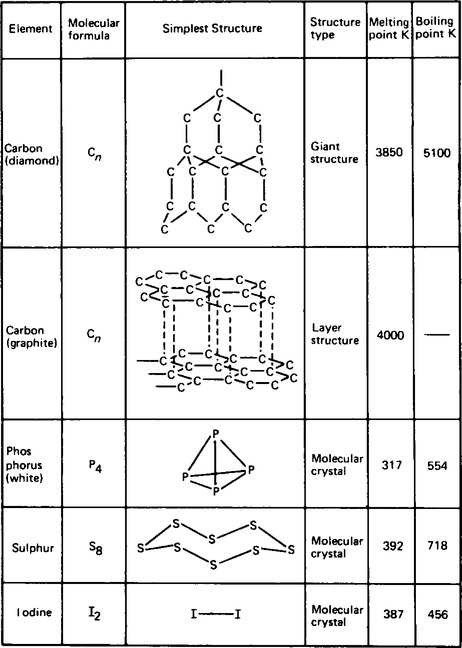
7. Some non-metallic elements are solids at room temperature and these include carbon, silicon, phosphorus, sulphur and iodine. The chemical bonding which occurs in these solid elements is either purely covalent, as in diamond (carbon) and silicon, or a combination of covalent bonding and extensive intermolecular forces of attraction (or van der Waal forces), as shown in Figure 56.3 by the layer structure of graphite and the molecular crystal structures of phosphorus, sulphur and iodine. The extent of the intermolecular forces can be established by the melting points of the respective solids as shown in Figure 56.3.