2
CURRENT CHALLENGES AND OPPORTUNITIES IN THE PHARMACEUTICAL INDUSTRY
Joseph L. Kukura
Merck Research Laboratories, Merck & Co., Inc., Rahway, NJ, USA
Michael P. Thien
Merck Manufacturing Division, Merck & Co., Inc., Whitehouse Station, NJ, USA
2.1 INTRODUCTION
The pharmaceutical industry bases its products, strategies, decisions, and actions and ultimately its very existence on the primary challenge of improving human health and the quality of life. The work of this industry uses a foundation of medical science to connect to the most basic struggle faced by all individuals and societies: the struggle for people to live healthy, productive lives. The industry has partnered with governments, health organizations, and society to achieve key successes in human history, including the eradication of smallpox, prevention of infection through the large‐scale production and distribution of antibiotics such as penicillin, and significant reductions in cardiovascular events. Advances in pharmaceutics have contributed to lower infant mortality rates and longer life spans observed over the past century. When considering the landscape of the pharmaceutical industry, one should retain the perspective that a challenge or opportunity that relates to the improvement of human health is at the core of challenges and opportunities shared by pharmaceutical companies.
2.2 INDUSTRY‐WIDE CHALLENGES
Just as life and the state of human health often undergo significant changes, the pharmaceutical industry is profoundly changing. Fundamental elements that molded past business models are dynamically moving into new realms in a manner that will challenge the continued vitality of pharmaceutical companies much in the same way that changes in environments can influence the health of people. The parallels between diseases that the industry seeks to address and the pharmaceutical business climate are distinctly apparent. Illnesses such as HIV/AIDS and cancer are more complex and evolve at a faster pace than many previous diseases, requiring new approaches and advances. Similarly, economic, societal, and scientific forces are rapidly driving changes to the industry's business models. The forces challenging the industry align into four categories: increased costs and risks, revenue/price constraints, globalization of activities, and increasing complexity of pharmaceutical science.
2.2.1 Increased Costs and Risks
Bringing a new medicine to market involves a long, complex process in a highly regulated industry. Estimating accurate or typical costs for successfully launching new pharmaceutical products is difficult. The estimates are a strong function of the success rates assumed for moving a program through various clinical trial stages. Estimates for the average cost to launch a pharmaceutical product ranged from $900 million to $1.7 billion in the early years of the twenty‐first century and rose to $2.6 billion over the following decade [1, 2]. Across the industry, costs are trending upward in a manner that forces business practices to adjust.
Developing new medicines for unmet medical needs always involved significant costs and risks. For every product brought to market, pharmaceutical companies have typically invested in several thousands of compounds during the drug discovery stage, hundreds of compounds in preclinical testing, and many (7–12) unsuccessful clinical trials over a period of 9–15 years, as depicted in Figure 2.1. Though the later‐stage phase II and phase III clinical trials are performed on the fewest number of compounds, they are also the most costly stages of development since they can require testing hundreds of patients in phase II and thousands in phase III in order to get statistically meaningful results. The cost of developing new medicines is therefore particularly sensitive to success rates of late‐stage clinical trials. The industry has generally experienced a decline in the fraction of compounds proceeding through phase II and phase III clinical trials to a successful regulatory approval and commercial launch of a new product. This decline translates to expending more resources on programs that do not return value on their investment and greater overall spending on research and development.

FIGURE 2.1 Number of compounds in research and development for every successful launch of a pharmaceutical product.
The lower clinical trial success rates are due in part from the fact that pharmaceutical companies are attempting to treat more complex therapeutic targets. Many diseases with straightforward cause–effect relationships and less sophisticated biological mechanisms have already been addressed, leaving more challenging and intricate problems for the future. The setbacks and frustrations relating to the development of treatments and vaccines for HIV infection serve as a case in point. Numerous articles and presentations have reported that the HIV virus mutates, adapts, and changes features faster than predecessors that were studied for vaccine development. Similarly, the causes of many forms of cancer being addressed in clinical trials have more complex physiological traits in comparison with successfully treated conditions such as high cholesterol and high blood pressure. Treating more complicated illnesses leads to higher risks for clinical trial evaluations.
The pharmaceutical industry research and development costs are also increasing due to greater regulatory hurdles for getting approval of new medication. Many agencies have raised their requirements for approval, leading to the need for larger and more comprehensive clinical trials and safety assessment testing. Government health agencies are showing a high level of caution with respect to side effects and risk–benefit assessments. This caution creates a need for outcome data and in turn longer running trials and longer review periods that can delay introduction of a product to market. A more conservative regulatory approach ultimately forces greater spending on development and testing of new medicines to collect the data needed for the higher standards.
2.2.2 Revenue/Price Constraints
Beyond the challenge of increasing costs, the pharmaceutical industry is also facing constraints to income and product pricing. The patents of large revenue “blockbuster” drugs are expiring faster than they are being replaced by a comparable portfolio of new highly profitable products. The challenges of addressing more complex therapeutic targets previously described in the context of increasing cost also directly affect revenue in the industry. The greater level of complexity not only makes the research and development process more expensive, but it also slows the realization of a return on investment in these areas. Many of these more sophisticated research efforts target a narrower patient base than preceding blockbusters. The largest sources of revenue for the industry in prior decades improved conditions that were widespread, such as depression, hypertension, and hypercholesterolemia. Far fewer people have conditions that many products currently in development aim to improve, such as specific forms of cancer. With a smaller base of potential patients, these new products can be expected to generate less revenue than broadly used products already on the market.
Another strong influence on pharmaceutical sales relates to the means by which patients pay for medicine. Organizations responsible to pay for prescriptions, the payers such as insurance companies and health maintenance organizations (HMOs), are influencing the medical options for their membership. The pharmaceutical companies used to be able to focus on physician–patient relationships when marketing products, but the decision‐making process to select medicine now involves a more complex set of interactions between physicians, patients, and payers. The pharmaceutical industry must engage all three members of this collective to successfully bring products to those who need them. Payers acquired an increasingly important role in this process in the United States through consolidations that have allowed a few groups to represent larger numbers of people. Single payers can control access to millions of patients [3]. Payers can exert their influence on the pharmaceutical industry in several ways. They cannot directly specify which medications a patient may use, but they can make co‐payments paid by patients much higher for some medications relative to others. If a payer wants to provide incentive for patients to request switching from a current treatment to a less expensive generic alternative, they can make the co‐payment for the generic version significantly less expensive. Similarly, payers can also choose to reimburse pharmacists at a higher rate for supplying generics and drive policies at pharmacies to favor the generic options.
In addition to consolidation of private payers, other events elevate the importance of payers to the pharmaceutical industry. The US government became effectively the largest payer to the pharmaceutical industry in January 2006 with the implementation of Medicare Part D prescription plan, covering over 39 million people with that plan alone [4]. Even more people will be eligible for coverage benefits in the coming years. If the US government alters its current policy to not negotiate medicine covered by Medicare or reimportation policies, the changes will create significant challenges to the business models of the pharmaceutical industry. The issue is certainly not limited to the United States. The changing demographics of the world will dramatically affect social medicine policies. The patients themselves in the patient–physician–payer relationship are changing in ways that will challenge the business models of the pharmaceutical industries. Across the world, the fraction of people above age 65 is growing as life expectancy increases. Never will the world have had this many people this old. Along with economically challenged populations in emerging non‐Western markets, this increasing fraction of the planet’s population will generally have limited income available for health care, but they will have a disproportionally strong demand for pharmaceutical products. Ensuring access to medicine across the globe and across population sectors will require lower prices. The pharmaceutical industry must adapt to meet the needs of these large segments of customers.
Not only are the demographics of patients changing, but their behaviors and approaches to health care are differing from the past. Survey results show that health care is a diverse consumer market with people seeking greater access to information to make their own choices with respect to health‐care needs [5]. With technology advances such as the internet, patients can get more information to play a larger role in selecting treatment options. In some parts of the world, direct advertising to consumers is prevalent and raising new levels of their awareness of options. Patients are also willing to explore innovative techniques or travel outside their area and even their country to find options that best suit their preferences. To face the revenue and price constraints introduced by patients’ actions to the patient–physician–payer relationship, pharmaceutical companies need to understand the changing manner in which patient behaviors affect market demand and pricing.
2.2.3 Globalization of Activities
To address financial constraints and meet the global demands of emerging markets, the pharmaceutical industry is increasing the activity levels of its business in these regions, moving away from being primarily located and focused in the Unites States and Europe. Like many other industries, a greater fraction of manufacturing and research and development is shifting overseas from a western base into countries such as China and India. Numerous clinical trials are conducted in these regions to achieve cost savings and to more quickly enroll patients who are not already undergoing another therapy. Development activities such as medicinal chemistry and process scale‐up are being performed there as well, leading to an expansion of sophisticated laboratories in these countries. Manufacturing is becoming increasingly well established in regions outside the United States and Europe, supplying global medical needs from truly global locations. Like international efforts in other industries, the globalization of pharmaceutical activities increases challenges associated with alignment of regulatory standards, logistics, language barriers, and cultural differences but pays dividends in cost reduction and increases in the size of the talent pool.
2.2.4 Increasing Complexity of Pharmaceutical Science
As already mentioned in the context of rising costs and constrained revenues, current research and development of new medicine is attempting to address afflictions and therapeutic categories that are more complex than their predecessors. In order to understand and treat these more complex targets, the industry must use more complex and difficult science. The pharmaceutical industry has always employed a highly talented collection of several scientific disciplines, ranging from biologists and chemists to engineers and statisticians. All of these professions now face harder problems down to the molecular level of their fields to bring forward the next generation of medicines. The scientific challenges take many forms. For example, a larger fraction of compounds in development have low solubility and low permeability in human tissue, making drug delivery within the body more difficult. Highly potent compounds dictate that the amount of drug in the formulation be very small, sometimes in the sub‐milligram ranges, and this also adds to the challenges of formulation development. Innovative and novel delivery systems are required to ensure new medicines are effective. Advances in the academic understanding of the workings of human genetic code are creating especially challenging questions around how to translate this knowledge into practical improvements in human health. Employees of pharmaceutical companies must be prepared for a future with more difficult challenges.
2.3 OPPORTUNITIES FOR CHEMICAL ENGINEERS
The challenges faced by the pharmaceutical industry create several opportunities for its members, including chemical engineers. The pressures to reduce costs connect directly to engineering principles that seek economies of scale and the application of efficient technology. In a related manner, chemical engineers can also reduce the lead time required to manufacture pharmaceutical products. Reducing lead time can have the parallel benefits of lowering inventory costs and protecting revenue by increasing the responsiveness of the supply chain to ensure changes in patient demands for medicine can be met. Technological innovation and engineering analysis can enhance products to create meaningful differentiation for patients in a variety of ways including contributions to drug metabolism and pharmacokinetics, which provides value in the face of revenue constraints. The complexities of pharmaceutical science and constraints of approaches that need to be suitable for global use are interwoven with the application of chemical engineering tools to address cost issues and enable product value. Finally, the strategic management of technology used to meet industry challenges by chemical engineers is an additional overarching opportunity in the industry.
Cross‐functional collaboration is vital to efforts to reduce costs, lower lead times, improve processes and products, and strategically manage new technology. Engineering alone cannot accomplish nearly as much as what can be achieved in combination with the organic chemists who design API synthetic routes, the analytical chemists who develop methods to measure the quality of the material, the formulators who design the final dosage form, the quality colleagues who help ensure processes are compliant, the operating staff who run the equipment, and the supply chain professionals who manage logistics. Chemical engineers are integrated into broad teams with a common goal to deliver the highest quality medicine to patients at the most affordable cost. To fully realize the more technical opportunities highlighted in this section, chemical engineers must engage in the opportunities to collaborate with a diverse group of professionals to learn from their perspectives and share innovative approaches to problem solving.
2.3.1 Reducing Costs with Engineering Principles
Owing to large margins, engineers in the nongeneric pharmaceutical industry historically did not have the same traditional focus on product cost as engineers in other businesses. With a renewed emphasis on cost, engineers are increasingly using a wide variety of engineering tools to improve costs and help maintain margins while maintaining high quality. These tools include modeling of unit operations, employment of efficient lab methods and design of experiments, combining the output of models and experiments to define advantageous processing options, and the use of standardized technology platforms. Some of these topics will be highlighted briefly here and discussed in greater detail in subsequent chapters.
Across many industries, engineers are employed to use process modeling and physical/chemical property estimation to maximize the yield and minimize the energy consumption and waste production associated with desired products. Using the broad applicability of this network of techniques is a continuing opportunity. Engineers in the pharmaceutical industry use computational tools originally created for oil refinery processes to optimize distillations and solvent recovery associated with the manufacture of active pharmaceutical ingredients (APIs) [6]. Similarly, thermodynamic solubility modeling can be applied to optimize crystallizations [7]. Computational fluid dynamics (CFD) has numerous applications to pharmaceutical flows [8]. The use of sound, fundamental chemical engineering science can eliminate bottlenecks, improve production, and unlock the full potential of biological, chemical, and formulation processes used to make medicine.
Chemical engineers can also use their training and expertise with technology to help reduce costs. In the R&D arena, the use of high‐throughput screening tools and multi‐reactor laboratory systems efficiently promotes the generation of data at faster rates. When modeling and estimation techniques cannot provide a complete picture, engineers can get the data they need quickly with high efficiency technology. It is important to recognize that not all of the advanced laboratory technology works universally well in all situations. A miniature reactor system suitable for homogeneous reactions may have insufficient mixing for heterogeneous chemistry. Selection of the appropriate laboratory technology and proper interpretation of results produced by these laboratory tools benefit from the perspective of combined chemical engineering principles such as mass transfer, heat transfer, reaction kinetics, and fluid mechanics. With the appropriate equipment in hand, engineers can utilize a statistically driven design of experiments to maximize the value of data generated via the experimental methodology.
The process understanding that comes from combining models and experimental data is a key opportunity for chemical engineers. Changes in the regulatory environment contribute to this opportunity. The advent of quality by design (QbD) principles (ICH Q8, Q9, and Q10) provides greater freedom after launching a product to modify operating parameters within a defined operating space. These changes can promote higher quality products and reduce process waste by applying the knowledge that comes with increased production experience once a medicine is commercialized. The modeling abilities of chemical engineers and their technology expertise will be able to provide crucial guidance to the definition and refinement of a QbD operating space. A thoughtful, well‐conceived operating space will in turn lead to long‐term gains in process efficiency and better results for consumers.
A primary method to achieve the benefits of chemical engineering principles at manufacturing scale comes from the development and application of technology platforms that can use a single set of equipment with common operating techniques across a portfolio of processes and products. The platforms not only reduce capital costs by allowing the purchase of a reduced amount of equipment for more applications, but development costs can also be lowered as well through a streamlined approach that comes from having a deep understanding and expertise with a technology platform. The familiarity and data obtained from running multiple projects in a single platform will translate into benefits for future projects that share common features. Broad uses of standardized platforms also make processes more portable for global applications. The key challenges of platforms are (i) knowing for which compounds the platform will be applicable and (ii) maintaining the knowledge gained about the platform and its underlying technology.
2.3.2 Reducing Lead Time
As the uncertainty and volatility of demand for pharmaceutical products increase, the value of reducing the lead time to manufacture medicine also increases. The lead time for ordering commodity raw materials for the first custom step in a synthesis to the release of an API can often take as long as 9–15 months, depending on the length and complexity of the synthesis, complexity of the supply chain, and availability of the required chemicals and manufacturing equipment. The lead time for the formulation of the API with excipients to make the drug product typically adds another two to six months, with a significant fraction of that time associated with the quality testing and release process.
The result of such long lead times is that companies will typically build various inventory levels of isolated intermediates, active ingredients, bulk drug product, and packaged products in order to fulfill unforecasted demand spikes that can arise during a replenishment lead time. The “safety stock” inventory level needs to be at least the replenishment lead time (LT) times the difference between the maximum demand rate (DRMax) pulled by the consumer that a company wants to be capable of meeting and the forecasted demand rate (DRFC) used for production planning:
By reducing lead time, the level of inventory to cover customer demand volatility can be reduced at least linearly. The gap between the maximum and forecasted demand rate may also decrease because forecasts are typically made with greater accuracy when the time between locking the forecast for production schedule and the final delivery is reduced [9]. Lead time reduction provides meaningful cost reduction because inventory costs include not only the warehousing costs to hold the material but also the interest costs of the money invested to manufacture the inventory. Money not spent on inventory builds can be invested elsewhere in the business. Further, if the actual demand rate is significantly lower than the forecasted demand, then material in the inventory can be vulnerable to expiring and needing to be retested, reprocessed, or discarded and replaced. A case study found that reducing the lead time from 10 months to less than 2 months for production of a vaccine can offset more than a 50% production unit cost increase, by reducing the vulnerability to discards stemming from uncertain demand [10]. Just as importantly, in cases where the actual maximum demand rate exceeds the level planned to be covered by inventory, having a shorter lead time can better meet the upside demands of the market and reduce the risks of stocking out of medicine needed by patients.
Engineers can reduce the lead time of production in a variety of ways. During development, process alternatives that improve reaction kinetics, reduce mass transfer restrictions, and enable more productive unit operations are fundamental to the engineering skill set and can lower lead times. However, the majority of the very long lead times for medicine do not involve the time required for material to change chemically or physically into a different form. Most of the lead time is commonly associated with holding material while waiting for it to be released for the next process step, transportation time between manufacturing sites, and/or waiting for the next available production window. Processes that follow multiple sequences of batch unit operations are especially vulnerable to such waiting periods. Engineers are strongly contributing to an increasing use of continuous manufacturing processes that inherently flow material from one production step to the next without building inventory of intermediates or pausing between transformations. Multiple types of processes and many unit operations are amenable to connecting steps that are segregated when run batchwise [11]. Continuous processes also lend themselves to integrating with process analytical technology (PAT) and other analytical tools to perform enhanced process control and real‐time release of material at intermediate stages of production. By integrating engineering principles with advanced analytical measurements and fundamental understanding of the associated organic and physical chemistry mechanisms, processes of the future will be able to flow through hold points that were necessary in the past.
2.3.3 Improving Product Value
Chemical engineers also have opportunities to meet market demands for pharmaceutical products that deliver greater value to patients, payers, and physicians. These customers generally do not care about the manufacturing process, but they do care about product convenience, safety, and compliance. They are seeking meaningful differentiation in these areas among their options. The remainder of this section will discuss ways engineers can contribute to product value with two examples: drug delivery and diagnostics.
Contributions to improvements in drug delivery vehicles serve as an excellent example of how engineers can improve pharmaceutical product features. The application of particle engineering and convection modeling to inhalers can improve the consistency with which a dose is administered via the respiratory system independent of the strength of the patient's breath [12]. Greater consistency of delivery increases the associated compliance. In orally administered capsules and tablets, engineers can manipulate polymer properties and transport driving forces to afford a consistent extended release of an API [13]. A steady, slow release of medicine from a single delivery vehicle can reduce both the frequency with which the medicine needs to be taken and potential side effects, which can, in turn, improve conveniences for the patient and compliance with the dosing regimen. In order to realize the benefits of controlled release, the pure API particle size distribution usually must be kept consistent prior to formulation. A great deal of engineering effort has been applied to maintain control of crystal sizes during the crystallization, filtration, and drying unit operations for drug substances [14].
Engineering principles can also be used to improve diagnostic tools used to treat diseases. Diagnostics are especially important for payer organizations that want to utilize options that have the highest probability of success for the patient. A diagnostic tool that enables physicians to initially assign the best treatment without going through a trial‐and‐error approach reduces the costs charged to the payers through the preemptive elimination of ineffective options. The aforementioned chemical engineering skills that aid the process of making medicines also contribute to improvements in making diagnostic technology. The underlying governing equations that characterize the transport of medicine to a specific target in the body also have applications in the movement of a sample from a patient through a device to the analysis component. Beyond diagnostic effectiveness, the ability for chemical engineers to respond to patient preference and improve the convenience of diagnostics tools used in the home or other areas outside of hospitals and physician offices is a key opportunity with health care becoming an increasingly consumer‐driven market [5]. Advances in polymer technology and manufacturing processes can lead to devices that are lighter, smaller, and more resilient to being dropped. Just like new models of an iPhone™ garner increased use over their heavier, larger, and more delicate predecessors, delivery vehicles and diagnostics serve as examples of significant opportunities for chemical engineers in the pharmaceutical industry to meet consumer demands for improved products.
2.3.4 Strategic Technology Management
In addition to direct scientific contributions that reduce costs and improve value for the pharmaceutical industry, engineers have the opportunity to help direct strategic investments in technology. Companies cannot afford to individually develop, implement, and advance all technologies required for their business. Several case studies show how good and poor strategies relating technology to business considerations have affected multiple industries, including computer companies and international distributors [15]. The availability of global development and supply options creates relatively new decisions for the pharmaceutical industry. Technology investment must be managed through a careful balance of internal capabilities, strategic partnerships, and reliance on external vendors. In order for this balance to be established and maintained, a holistic definition and view of technology must first be established. Is any laboratory or production device such as a granulator or a blender considered technology, or are they just pieces of equipment? In the context of strategic management, technology can be defined as a system composed of (i) technical knowledge, (ii) processes, and (iii) equipment that is used to accomplish a specific goal. The knowledge encompasses the understanding of fundamental principles and relationships that provide the foundation of the technology. The processes are the procedures, techniques, and best practices associated with the technology. The equipment is the physical manifestation of the technology such as devices, instruments, and machinery. The goal for strategic technology management is to make value‐driven decisions around investments in the advancement, capacity, and capability with each of the technology components.
To make those investment decisions, industrially relevant technology can be assigned to categories. The concepts of core technology and noncore technology and associated subcategories are useful in this regard:
- Core technology: Core technology is sophisticated technology that makes critical contributions to the core business. As such, it justifies investment in all three components of the technology (knowledge, process, equipment) to afford a competitive advantage. Options for core technology include maintaining internal capability for all three components as a primary technology or managing the technology via external capacity as a partnered technology. The distinguishing feature of all core technology is that internal capability in the knowledge component of the technology is typically required to ensure the technology is adequately controlled to meet core business needs:
- Primary technology: Primary technology is a category of core technology that offers competitive advantage by maintaining internal capability for the complete technology system. The investment in internal capacity does not need to meet all business uses of the technology but ensures that adequate resources can be provided for critical projects.
- Partnered technology: Partnered technology is a technology that contributes to the core business, but the company can maintain a competitive advantage while relying on external partners to be primarily responsible for parts of the technology system. The company may invest in knowledge and process development for a partnered technology while utilizing external equipment capacity and potentially outside process expertise.
- Noncore technology: Noncore technology does not warrant investment or control in all three components of the technology system. The subcategories of emerging technology and commoditized technology characterize the most relevant noncore technology:
- Emerging technology: Emerging technology has potential to contribute significant business value in the future but generally requires additional investment in the knowledge base before it can be applied in practice to the core business. Emerging technology is not necessarily brand new technology, but its application to the core business may be atypical or speculative.
- Commoditized technology: Commoditized technology is mature technology that is reliable, well established within the industry, cost efficient, and available in the market such that little investment in the technology is required.
To determine if a technology of interest is core or noncore, the connection of the technology to business value must be assigned as well as risks associated with the technology's ability to meet business requirements. Business value (BV) can be determined by identifying the revenue enabled by products made via the technology. Risk (R) can be calculated or estimated by assessing the fraction of attempts that a technology fails to deliver intended results within predetermined specifications for both quality and efficiency. A minimum business value (BVmin) and minimum risk tolerance (Rmin) for being a core technology should then be assigned based on a strategic business and financial perspective. If either the business value or risk associated with a technology is below the corresponding minimum, the technology should not be considered for core technology investment. When a technology meets the minimum risk and business value requirements, a threshold value (TV) can serve as the primary criteria for determining the core technology designation as follows:
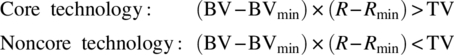
The underlying principle of this approach is that the core technology investment ensures the value benefit of the technology to the core business and mitigates the risk of a severe failure in the application of the technology. This insurance and mitigation come from the direct investment and maintenance of expertise in all three components of the technology, whereas noncore technology takes more appropriate risks with lower investments. As technology evolves in importance and reliability, it can transition between the core and noncore regimes by regular assessment of the business value and risk associated with the technology.
Technology progression and the evolution of a concomitant investment approach can be illustrated graphically on a plot of business value versus risk. On such plots, core technologies fall into the upper right regions. Risk generally decreases as time progresses and experience with the technology increases. A life cycle thus moves from right to left on the value–risk plot, and two examples are shown in Figure 2.2. In both cases, the technologies start with a relatively high risk as emerging technologies and transition from noncore to core technology when the business value becomes sufficiently high. In case 1, the technology sustains business value long enough for the technology to become a low risk commoditized technology. The business value remains high as the risk with the technology decreases, and the reduced risk drives the transition from core to noncore technology in this case. Technologies that make sterilized vials serve as an example here. At one point, the pharmaceutical industry needed to invest internal resources to ensure vials for vaccines would be sterile, but they are now readily available as a commodity made from reliable, established technology managed by vendors. In contrast, case 2 illustrates a decreased business value driving the transition from core to noncore technology, perhaps due to the introduction of a better replacement. Obsolete open‐top vacuum funnel filters that have been replaced by centrifuges and sealed filter dryers in manufacturing environments to improve industrial hygiene and efficiency provide an industrial example of case 2. Technologies relating to crystallization, spray drying, and roller compaction are representative of current chemical engineering core technology at several pharmaceutical companies.
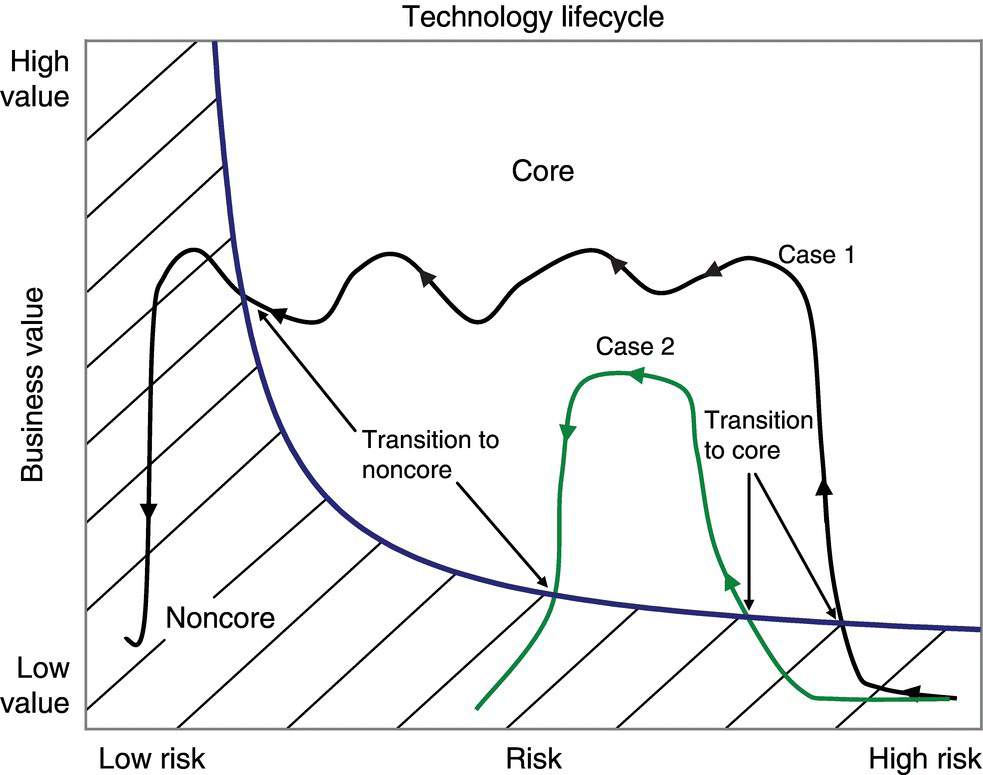
FIGURE 2.2 Examples of progression along a technology life cycle between core and noncore regimes.
Engineers have opportunities to substantially contribute to several facets of technology management as outlined above. Due to the complex nature of pharmaceutical processes, assigning a failure event to specific technology can be a challenging multivariant problem during risk assessment and risk management endeavors. Fundamental process understanding and technology expertise is vital to evaluating quantitative contributions to risk. Similar skills are useful for objectively determining whether value is enhanced through the use of internal capabilities versus external options. The understanding of a technology is important for determining the reliability of a prospective partner using that technology for critical business needs. Engineers also can contribute to investment choices among various emerging technologies with technical assessments of probabilities of success and potential applicability across a company's portfolio of products.
2.4 PROSPECTS FOR CHEMICAL ENGINEERS
Chemical engineers have made enabling contributions to health care, which serve as a strong foundation for future success. However, the pharmaceutical industry is profoundly changing, and the role of engineers must change with it. The industry challenges described in the second section of this chapter translate into the opportunities for chemical engineers in the third section. The use of modeling, standardized technology platforms, and a sound technology strategy will allow engineers to help reduce costs and lead times. Platform technologies also will assist with the challenges of making processes portable in an era of globalization. The need for greater product value to enable future revenues can partially be met by engineering enhancements to delivery devices and diagnostic tools. Additionally, engineers may also be able to improve the stability of formulated products, thereby reducing the need for expensive cold storage and enhancing access options for patients in severe environments. As the underlying science and supporting academic chemical engineering research evolve toward an increasing molecular basis, the perspectives and training of engineers must move from macroscopic and continuum foundations to a combined macroscopic, continuum, and molecular view. Chemical engineering will continue to integrate with the rest of scientific disciplines beyond a confined role in processing realms. The work of engineers must progress beyond connecting process contributions to production efforts to integrating the processes with the product itself. These efforts will be performed in the context of changing business models and pricing constraints on an increasingly global stage.
REFERENCES
- 1. Gilbert, J., Henske, P., and Singh, A. (2003). Rebuilding Big Pharma's business model. In Vivo: The Business and Medicine Report 21 (10): 73–80.
- 2. Mullin, R. (2014). Tufts study finds big rise in the cost of drug development. Chemical and Engineering News 92 (47): 6.
- 3. Steiner, M., Bugen, D., Kazanchy, B. et al. (2007). The continuing evolution of the pharmaceutical industry: career challenges and opportunities. Regent Atlantic Capital and Fiduciary Network, December 2007.
- 4. New Tech Media (2007). Medicare drug program hits 39 million, still open for advantage plans. Senior Journal.com, January 2007.
- 5. Deloitte Center for Health Solutions (2008). Opportunities for life science companies in a consumer‐driven market. www.deloitte.com (accessed 2009).
- 6. Li, Y.‐E., Yang, Y., Kathod, V., and Tyler, S. (2009). Optimization of solvent chasing in API manufacturing process: constant volume distillation. Organic Process Research and Development 13 (1): 73.
- 7. KoKitkar, P., Plocharczyk, E., and Chen, C.‐C. (2008). Modeling drug molecule solubility to identify optimal solvent systems for crystallization. Organic Process Research and Development 12 (2): 249–256.
- 8. Kukura, J., Arratia, P., Szalai, E. et al. (2002). Understanding pharmaceutical flows. Pharmaceutical Technology 26 (1): 48–73.
- 9. de Treville, S., Schurhoff, N., Trigeorgis, L., and Avani, B. (2014). Optimal sourcing and lead‐time reduction under evolutionary demand risk. Production and Operations Management 23: 2103–2117.
- 10. de Treville, S., Bicer, I., Chavez‐Demoulin, V. et al. (2014). Valuing lead time. Journal of Operations Management 32: 337–346.
- 11. Cole, K., Groh, J.M., Johnson, M. et al. (2017). Kilogram scale prexasertib monolactate monohydrate synthesis under continuous‐flow cGMP conditions. Science 356: 1144–1150.
- 12. Finley, W.H. (2001). The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction. New York: Academic Press.
- 13. Wise, D. (2000). Handbook of Pharmaceutical Controlled Release Technology. New York: CRC.
- 14. Tung, H.‐H., Paul, E.L., Midler, M., and McCauley, J. (2009). Crystallization of Organic Compounds: An Industrial Perspective. Hoboken, NJ: Wiley.
- 15. Shimizu, T., Carvalho, M.M., and Laurindo, F.J.B. (eds.) Concepts and history of strategy in organizations. In: Strategic Alignment Process and Decision Support Systems: Theory and Case Studies, 2006. Hershey, PA: Idea Group Publishing.
