48
SCIENTIFIC OPPORTUNITIES THROUGH QUALITY BY DESIGN
Timothy J. Watson and Roger Nosal
Global CMC, Pfizer, Inc., Groton, CT, USA
Quality by design (QbD) is about understanding the relationships between the patient’s needs and the desired product attributes by ensuring that all process attributes and parameters that are functionally related to safety and efficacy are consistently met. The value of proactively gaining enhanced process knowledge and understanding significantly minimizes patient risk while balancing continued product improvements, innovation, business needs, and regulatory oversight. It becomes the foundation that links research and development, manufacturing, and regulatory agencies through a fundamental common language that is based on a science‐ and risk‐based philosophy.
For decades, much of the activity in quality and quality management was placed on compliance rather than utilizing an understanding of the science. Business practices adapted to procedures and focused on minimizing regulatory risk. The implementation of new technology was not typically part of a strategy because oversight for novel technology was not precedented. Extremely risky and high attrition rates of research programs, unlike any other industry, coupled with the lack of global harmonization between agencies, significantly challenged investing in new technologies and using modern methodologies for development and in submissions. In the 1990s the use of PAT started to gain interest in pharmaceutical manufacturing; however, it was primarily used for business purposes and not seriously considered for regulatory purposes. Describing to regulatory authorities a comprehensive view of process understanding was avoided in fear of being held accountable and overregulated.
In August 2002, the FDA launched their GMPs for the twenty‐first‐century initiative in partial response to academics and consultants, indicating the pharmaceutical industry was not manufacturing to very high standards. Companies were encouraged to use risk‐based assessments, in particular when identifying product quality attributes, and adopt integrated quality systems throughout the life cycle of a product. This movement toward science‐based regulations has not been limited to the United States as seen by the guidance provided by the International Conference on Harmonization (ICH). Thus, QbD for the pharmaceutical industry evolved from a vision to obtain a mutually efficient, agile, flexible sector that reliably produces high quality drug products without extensive oversight.1 Guidance for QbD was crafted through the ICH process to what is now considered the “QbD trio”: ICH Q8, Q9, and QlO (ICH Qll for drug substance in draft as of 2009). This movement away from prescriptive development programs has become an exciting and empowering platform for chemists, scientists, formulators, and engineers. While many elements associated with QbD, i.e. risk assessments, design of experiments (DoE), operational control strategies, etc., have been employed well before the adoption of the ICH guidelines, application was frequently not systematic, concerted, or prospective, but rather retrospective in response to issues or problems encountered during development or after commercial launch. Consequently, companies were reluctant to pursue a QbD approach or introduce supplemental studies on process capability for fear of unnecessarily increasing regulatory “burden” and potentially delaying regulatory approvals.
QbD begins with a prospective vision that accepts and builds upon a science‐ and risk‐based platform with a commitment to never lose sight of customer focus. It starts with the quality target product profile (QTPP) and works backward through the drug product and drug substance platforms to establish a holistic understanding of what attributes are linked to patients’ needs and how these attributes are functionally related through the entire process. Ensuring patient safety is not about “what we apply” to maintain the QTPP, but it is about “how we apply” process understanding, and it is about “what we need and what do we have” to ensure that quality is consistently met. It is about identifying the relationship between each attribute and its manufacturing origin and consistently controlling these relationships [1].
A drug product or drug substance control strategy should describe the functional relationship between drug product/drug substance critical quality attributes and material attributes and process parameters that govern the manufacture and effectively demonstrate control of quality. It is generally recognized that the fundamental concept of QbD is control strategy, and ICH was clear to state that every CQA has a control strategy, where concepts like design space could be (but not mandatory) an element for the control chosen for any given CQA. The development of potential design spaces as an element of the control strategy is a symbiotic relationship that encompasses all of the concepts contained within the chapters of this book. In adopting a QbD approach and applying the science‐ and risk‐based principles to assess quality attributes and process parameters, design space can be created to describe the boundaries within which unit operations of a manufacturing process may operate. In essence, design space can demonstrate control of variables that may impact a critical quality attribute, and a control strategy can be established to accommodate design space. For example, a combination of well‐defined design space boundaries and real‐time release testing can effectively demonstrate and confirm control and serve as the basis for release of the product without the need for specific end‐product testing. Therefore, where the risk is understood and the severity and probability of impact are controllable, the demonstration of process control through the creation of design space could conceivably reduce the need to perform in‐process testing as well. Continuous formal verification to demonstrate process capability in accordance with well‐grounded design space criteria could serve as the basis for product release to a specification derived largely from critical quality attributes (Figure 48.1).
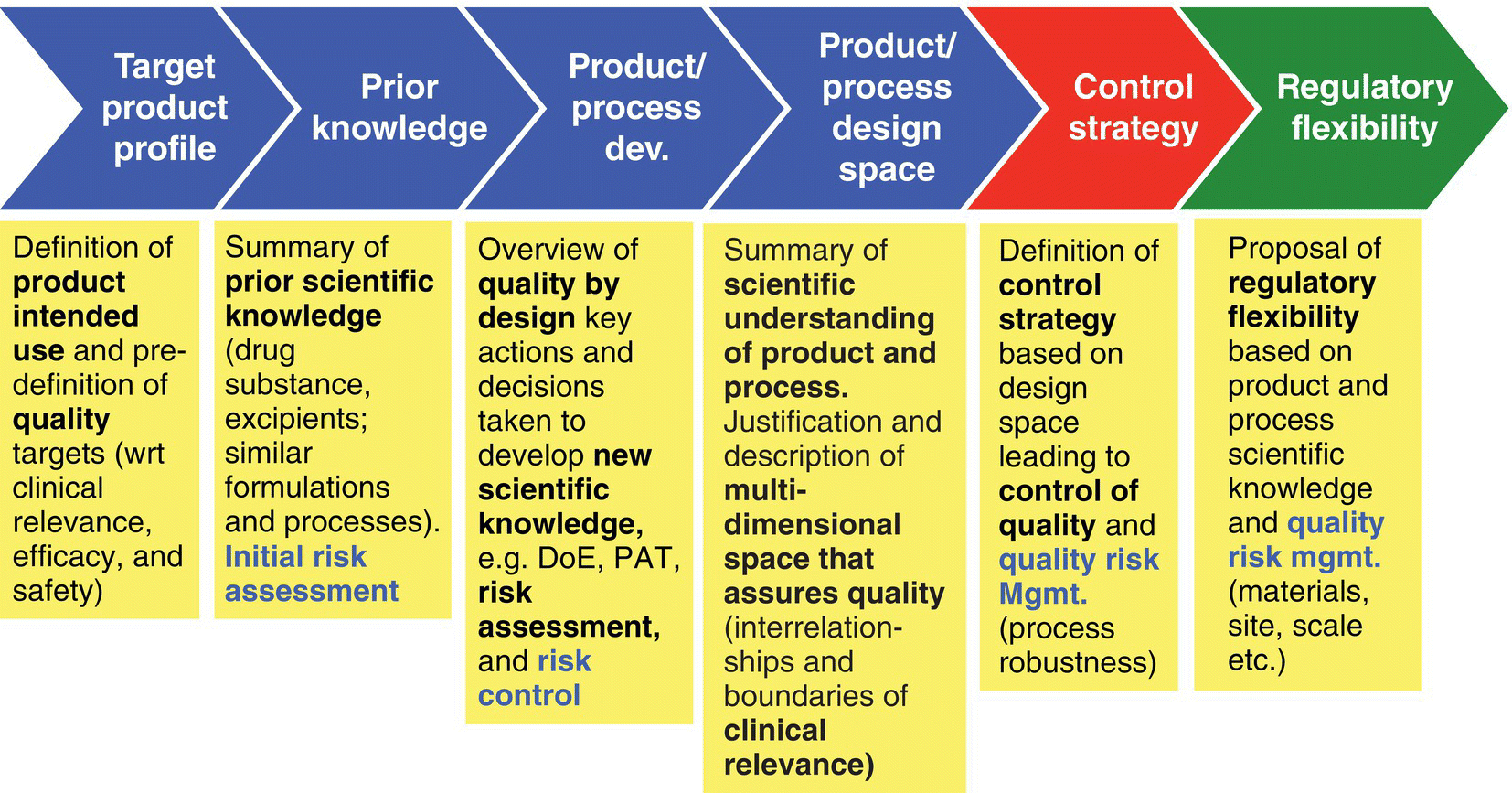
FIGURE 48.1 General outline of approach to application of quality by design.
Scientist that embraces an enhanced approach to development should consider these types of questions:
- How is prior knowledge substantiated, and how can internal and external knowledge be used to leverage more accurate risk assessments?
- What level of detail is required to justify risk assessments?
- How should design space be presented and conveyed to demonstrate quality assurance?
- How can modeling be used to justify commercial manufacturing process changes?
- How should the control strategy connect drug product and drug substance quality attributes to process parameters and material attributes?
- Is there an attenuation of regulatory latitude for post‐approval optimization and continual improvement?
In addition, there are many general processes that can be adapted to sketch out a general procedure for any team to adapt a science‐ and risk‐based approach. One example is given in Figure 48.2. Common themes to any of these processes are to constantly perform risk assessments on the attributes/parameters and their connectivity to the QTPP, use an iterative approach to risk and experimental data evaluation, be creative to understand parameter interaction in a multivariate process, present a solid design space and control strategy to ensure safety and quality are top priority, and be open and transparent to ensure the data generated can stand peer review and “pass the red face test.” Far from suppressing progress, these, among many other questions, stimulated regulatory authorities and industry to pursue clarification. A fair measure of subsequent progress has improved the consistent application and value of these concepts.

FIGURE 48.2 Small example of drug substance workflow for QbD.
The intrinsic advantages of investing in process understanding increase confidence and assurance of product quality. Tangible benefits, reductions in manufacturing costs associated with improved efficiencies and expeditious innovations, and reduction in manufacturing recalls, failures, or extraneous investigations attributed to uncertainty are largely realized over the long term, as the life cycle of a product matures.
The fundamental scientific premise as a result of QbD that attracts scientific support from every discipline across this industry is driven by a common passion to develop improved process understanding and product knowledge. The movement away from prescriptive development programs has become an exciting and empowering platform for chemists, scientists, formulators, and engineers. QbD has also played an instrumental role in establishing the value and importance of cross‐functional, scientific relationships in pharmaceutical development through proactively developing and understanding processes and formulations. Perhaps, most importantly, the application of a QbD approach and investment in robust pharmaceutical quality systems are expected to reduce unexpected variability in manufacturing processes and unanticipated failures in product quality, thereby improving quality assurance of products.
ACKNOWLEDGMENT
The authors would like to thank Robert Baum from Pfizer for sharing his knowledge and thoughts on the historical perspective on QbD.
REFERENCE
- 1. Watson, T.J.N., Nosal, R., am Ende, D. et al. (2007). API quality by design example from the torcetrapib. Journal of Pharmaceutical Innovation 2 (3–4): 71.
