Chapter 18
All about Batteries
Forget miniature atomic piles. Forget dilithium crystals. The robots in science fiction are seldom like the robots in real life. With few exceptions, today’s robots run on batteries—the same batteries that power a flashlight, portable CD player, or cell phone. To robots, batteries are the elixir of life, and without them, robots cease to function.
To be sure, batteries may not represent the most exciting technology you’ll incorporate into your robot. But selecting the right battery for your bot will go a long way toward enhancing the other parts that are more interesting. Here’s what you need to know.
An Overview of Power Sources
Before getting waist-deep in the big muddy of battery selection, let’s first review the practical power sources available for use with mobile robots. Note the word practical, m’kay? There are plenty of potential power sources available in the world. Some forms of power are not suitable because of their size, safety, or cost.
![]() Windup mechanisms provide power using tension that is slowly released. A common type is based on the idea of a clock mainspring. These use a metal coil as a tension spring. The coil powers a shaft or other movement as its tension is relieved. For robotics, the typical windup mechanism is confined to small toys, particularly older collectable toys.
Windup mechanisms provide power using tension that is slowly released. A common type is based on the idea of a clock mainspring. These use a metal coil as a tension spring. The coil powers a shaft or other movement as its tension is relieved. For robotics, the typical windup mechanism is confined to small toys, particularly older collectable toys.
![]() Solar cells get their power from the sun and other light sources. A disadvantage of solar cells is that power is directly related to the intensity of the light. Robots that use solar power are often equipped with a rechargeable battery or a large capacitor; both store the energy collected by the solar cell for later use.
Solar cells get their power from the sun and other light sources. A disadvantage of solar cells is that power is directly related to the intensity of the light. Robots that use solar power are often equipped with a rechargeable battery or a large capacitor; both store the energy collected by the solar cell for later use.
![]() Fuel cells are gaining in popularity as an alternative energy source. Most use hydrogen in a complex chemical reaction that produces heat, as well as a flow of electricity between two electrodes.
Fuel cells are gaining in popularity as an alternative energy source. Most use hydrogen in a complex chemical reaction that produces heat, as well as a flow of electricity between two electrodes.
Figure 18-1 Desktop robot (this one uses tracks) hides its battery supply under an expansion panel. There’s room for additional batteries (as needed) or a larger battery holder.
![]() Batteries are by far the most common and among the least expensive methods of powering any mobile device. Batteries can be grouped into two broad categories: nonrechargeable and rechargeable. Both have their place in robotics, and cost and convenience are the primary factors dictating which to use. These issues are discussed throughout the chapter. Figure 18-1 shows a robot and its power source—ordinary household batteries in a convenient holder.
Batteries are by far the most common and among the least expensive methods of powering any mobile device. Batteries can be grouped into two broad categories: nonrechargeable and rechargeable. Both have their place in robotics, and cost and convenience are the primary factors dictating which to use. These issues are discussed throughout the chapter. Figure 18-1 shows a robot and its power source—ordinary household batteries in a convenient holder.
Batteries for Your Robots
While there are hundreds of battery compositions, only a small few are ideal for amateur robots.
CARBON-ZINC
Carbon-zinc batteries are also known as garden-variety “flashlight” cells: because that’s the best application for them—operating a flashlight. They’re an old technology and not up to the task of running a robot. Let’s move on.
ALKALINE
Alkaline batteries (not to be confused with Hall-of-Famer Al Kaline of the Detroit Tigers) offer several times the operating capacity of carbon-zinc and are the most popular nonrechargeable battery used today. Robotics applications tend to discharge even alkaline batteries rather quickly, so a bot that gets played with a lot will run through its fair share of cells. Good performance, but at a price.

Alkalines are also available in a super-duper form; these go by a variety of self-descriptive names, like Monster and Ultra. High-capacity alkalines are made for loads demanding higher power. They’re pretty expensive, though, making them best suited as emergency backup power, in case your regular robo batteries get unexpectedly worn out.
RECHARGEABLE ALKALINE
Rechargeable alkaline batteries are the mass-merchandizing answer to the high cost of regular alkaline batteries used in high-demand applications—robotics is certainly one such application, though battery makers had things like portable CD players in mind when they designed these puppies. Rechargeable alkalines require a recharger designed for them and can be revived dozens or hundreds of times before discarding.
Rechargeable alkalines are probably the best choice as direct replacements for regular alkaline cells. The reason: Most rechargeable batteries put out about 1.2 volts per cell; both rechargeable and nonrechargeable alkalines are rated at 1.5 volts per cell. See “Understanding Battery Ratings,” later in this chapter, for more details about cell voltage.
NICKEL-CADMIUM
Nickel-cadmium rechargeable batteries are an old technology and, unfortunately, one that’s unfriendly to the environment—cadmium is extremely toxic. Lately, battery companies have been favoring the “greener” nickel-metal hydride formulation that follows. Nickel-cadmium batteries are still plentiful, and you’ll likely use them for at least some of your projects.
Nickel-cadmium (NiCd for short) cells are available in all standard sizes, plus special-purpose “sub” sizes for use in sealed battery packs for consumer products—things like rechargeable handheld vacuum cleaners, cordless phones, and so forth. Most battery manufacturers claim their NiCd cells last for a thousand or more recharges.
A new, higher-capacity NiCd battery is available that offers two to three times the service life of regular nickel-cadmium cells. More important, these high-capacity cells provide considerably more power and are ideally suited for robotics work. Of course, they cost more.
Earlier NiCd batteries suffered from “memory effect,” whereby the useful capacity of the battery was reduced the more times it was recharged. The newer NiCd batteries—those made within the past 10 years or so—are said not to exhibit this memory effect, or at least not as much as the older variety.
NICKEL METAL HYDRIDE
Nickel metal hydride rechargeable batteries not only offer better performance than NiCds, they don’t make fish, animals, and people (as) sick when they are discarded in landfills. They are the premier choice in rechargeable batteries today, including robotics, but they’re not cheap. They require a recharger made for them (Figure 18-2). Many of the latest rechargers will work with rechargeable alkalines, NiCds, and NiMHs; just don’t use a NiCd-only recharger with NiMH.
NiMH batteries can be recharged 400 to 600 times and have what’s known as a low internal resistance. That means they can deliver high amounts of juice in a short period of time. Unlike NiCds, NiMH batteries of any type don’t exhibit a memory effect. NiMH cells can’t be recharged as many times as NiCd batteries: about 400 full charge cycles for NiMH, as opposed to 2000 cycles for NiCd.
LITHIUM-ION
Lithium-ion cells are frequently used in the rechargeable battery packs for laptop computers and high-end camcorders. They are the Mercedes-Benz of batteries and are surprisingly light-weight for the current output they provide. However, Li-ion cells require specialized rechargers; both battery and recharger can be frightfully expensive.
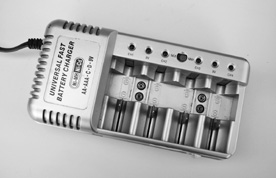
Figure 18-2 This universal charger works with both NiCd and NiMH batteries. You flip a switch depending on which kind you are recharging.

Figure 18-3 Sealed lead-acid (SLA) batteries are the largest and heaviest of the bunch, but they provide a lot of current, useful for larger robots.
SEALED LEAD-ACID (SLA)
Sealed lead-acid batteries are similar to the battery in your car, and they work in much the same way. SLAs are “sealed” to prevent most leaks, but in reality the battery contains tiny pores to allow oxygen into the cells. SLA batteries, which are rechargeable using simple circuits, are the ideal choice for very high current demands, such as battle bots or very large robots.
They’re also pretty inexpensive for the amount of juice they pack. However, they are among the heaviest of all the rechargeables, so use them only in robots that can support the weight.
SLA batteries are most often sold in 6-and 12-volt packs, like the one in Figure 18-3 (24-volt and higher packs are also available, but not quite as common). Inside the battery are multiple 2-volt cells—the cells are combined to create the desired voltage. Three cells are used to make a 6-volt pack, for example.
SO WHICH ONE SHOULD I PICK?
Most experienced builders select from a small palette of battery types based on the size and application of their robot.
![]() Alkaline, NiCd, and NiMH batteries are the most common among small tabletop robots. When using alkalines, you may chose between the rechargeable and nonrechargeable types. Nonrechargeable alkalines are a convenience, but using them can be expensive if you need to replace them often. Opt for rechargeable alkalines to save some money, or switch to NiMH or NiCd cells.
Alkaline, NiCd, and NiMH batteries are the most common among small tabletop robots. When using alkalines, you may chose between the rechargeable and nonrechargeable types. Nonrechargeable alkalines are a convenience, but using them can be expensive if you need to replace them often. Opt for rechargeable alkalines to save some money, or switch to NiMH or NiCd cells.
![]() Midsize “rover” robots use larger NiMH or Li-ion cells; bigger robots still are ideally suited for sealed lead-acid batteries. SLA batteries are available in a wide variety of capacities, and the capacity largely determines the size and weight of the battery. More about battery capacity in a bit.
Midsize “rover” robots use larger NiMH or Li-ion cells; bigger robots still are ideally suited for sealed lead-acid batteries. SLA batteries are available in a wide variety of capacities, and the capacity largely determines the size and weight of the battery. More about battery capacity in a bit.
![]() Because of the power demands, larger robots, such as those for machine combat, use sealed lead-acid batteries whenever possible. The biggest and most brutish robots can use liquid electrolyte batteries originally intended for use in motorcycles, small boats, or cars. These batteries are the heaviest of the bunch, but they pack a lot of wallop.
Because of the power demands, larger robots, such as those for machine combat, use sealed lead-acid batteries whenever possible. The biggest and most brutish robots can use liquid electrolyte batteries originally intended for use in motorcycles, small boats, or cars. These batteries are the heaviest of the bunch, but they pack a lot of wallop.

Which is better for the environment: nickel-cadmium or nickel-metal hydride? Actually, both contain poisonous materials, and both pose a threat. Never throw away your batteries, no matter what they’re made of. Recycle instead. If you have a choice, NiMH batteries are probably the best bet, but remember to recycle those, too.
Understanding Battery Ratings
Batteries carry all sorts of ratings and specifications. The two most critical are voltage and capacity.
VOLTAGE
The importance of voltage (or V for short) is obvious: the battery must deliver enough volts to operate whatever circuit it’s connected to. A 12-volt system is best powered by a 12-volt battery. Lower voltages won’t adequately power the circuit, and higher voltages may require voltage reduction or regulation, both of which entail some loss of efficiency.
Nominal (“Normal”) Voltage Level
Battery voltage is not absolute. The voltage of a battery may—and usually does—diminish as it is used.
Take a battery that’s rated at 1.5 volts. It puts out 1.5 volts, give or take. That “give or take” is important; the rated voltage of a battery may vary as much as 10 to 30 percent. When fully charged, the typical 1.5-volt cell may deliver 1.65 volts. When fully discharged, the voltage may drop to 1.2 volts.
Batteries are rated at a nominal voltage (see Figure 18-4). Nominal simply means “normal.” Only for a certain period during the battery’s discharge does it actually deliver this specific voltage.

Figure 18-4 Simplified but representative discharge curves for several popular types of batteries used in robotics: NiCd, NiMH, alkaline, and lead-acid.
To achieve higher voltages, you can link cells together, like lights on a Christmas tree. See “Increasing Battery Ratings,” later in this chapter, for more details. By linking together six 1.5-volt cells, for example, your battery “pack” will provide 9 volts.
When Decreasing Voltage Becomes a Problem
The varying voltage of a battery as it discharges doesn’t usually present a problem. That is, unless the voltage falls below a certain critical threshold. That depends on the design of your robot, but it usually affects the electronic subsystems the most.
Batteries are considered dead when their power level reaches about 70 to 80 percent of their rated voltage. That is, if the cell is rated at 6 volts, it’s considered “dead” when it puts out only 4.8 volts. Some equipment may still function below this level, but the efficiency of the battery is greatly diminished.
Most electronics systems in robots use a voltage regulator of some type, and this regulator requires some overhead … usually a volt or two. As the battery voltage drops below that needed for the regulator, the electronics go into a “brownout” mode, where they still receive power but not enough for reliable operation.
Brownouts are a common and damnable problem in robotics. You want to avoid this scourge at all costs. See Chapter 19, “Robot Power Systems,” for some ideas. If your robot has an onboard computer, you want to avoid running out of juice midway through some task. At best, this is a nuisance; at worst, damage to the robot or its surroundings could occur. See Chapter 19 for ways to add a simple battery voltage monitor to your bot.
CAPACITY
A common analogy for explaining electricity is to compare it to water going through a pipe. If voltage is pressure, then current is the amount of water that flows through every second.
Current in a battery determines the ability of the circuit it’s connected with to do heavy work. Higher currents can illuminate brighter lamps, move bigger motors, propel larger robots across the floor, and at higher speeds.
Because batteries cannot hold an infinite amount of energy, the current of a battery is most often referred to as an energy store, and is also referred to as capacity, abbreviated C.
Capacity Expressed in Amp-Hours
Battery capacity is rated in amp-hours, or roughly the amount of amperage (a measure of current) that can be delivered by the battery in a hypothetical one-hour period. In actuality, the amp-hour rating is an idealized specification: it’s really determined by discharging the battery over a 5-to 20-hour period, as shown in Figure 18-5.
What exactly does the term amp-hour mean? Basically, the battery will be able to (again, theoretically) provide the rated current for 1 hour. This current is expressed in amps—short for amperes—the common unit of expressing current flow from one part of an electrical circuit to another. (Technically, 1 amp is equal to about 6.24 × 1018 electrons passing by in 1 second. Pity the poor guy who had to count all those electrons.)
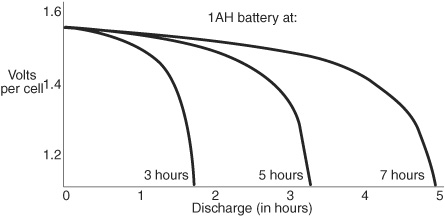
Figure 18-5 While batteries carry an “amp-hour” rating, it is actually tested under a lighter current load, for a longer period of time. Battery discharge at different current rates allows the battery to last longer.
If a battery has a rating of 5 amp-hours (expressed as “Ah”), the battery can—theoretically—provide up to 5 amps continuously for 1 hour, 1 amp for 5 hours, and so forth.
The battery is used for 10 or 20 hours, at a fairly low discharge rate. After the specified time, the battery is tested to see how much capacity it has left. The rating of the battery is then calculated by taking the difference between the discharge rate and the reserve capacity and multiplying it by the number of hours under test.
Plan for Extra Capacity
When choosing a battery, select one that has a capacity of at least 40 percent (preferably more) than the highest current demand of your robot. Design the robot with the largest battery you think practical. If you find that the battery is way too large for the application, you can always swap it out for a smaller one. It’s harder to do the reverse.
Some components in your robot may draw excessive current when they are first switched on, then settle to a more reasonable level. Motors are a good example of this. A motor that draws 1 amp under load may actually require several amps at start-up, as shown in Figure 18-6. The period is very brief, on the order of 100 to 200 milliseconds.
The Dangers of Overdischarging
As a battery discharges, it produces heat. Not only is heat destructive to batteries (and, therefore, heat production may be intentionally limited by the design of the battery), but it alters the electrical characteristics of them.
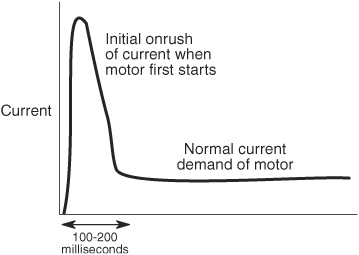
Figure 18-6 Current demand is the highest when electronic components, especially motors, are first switched on. The high onrush of current lasts a fraction of a second but can cause problems for the robot’s electronics.
The faster the discharge, the higher the heat that is generated. With few exceptions, batteries are not engineered to dump all their current in a short period of time. So manufacturers provide an idealized specification that more accurately represents the typical use of their wares.
Capacity Ratings for Smaller Batteries
Smaller batteries are not capable of producing high currents, and their specifications are listed in milliamp-hours. There are 1000 milliamps in 1 amp. So a battery that delivers half an amp is listed with a capacity of 500 milliamp-hours, abbreviated mAh (or less accurately as mA).
Even larger batteries might be rated in milliamp-hours, as is the case with rechargeable NiCd and NiMH cells, where it’s not uncommon to see them listed as 2000 mAh—that’s the equivalent of 2 amp-hours. Amp-hour ratings are typical in sealed lead-acid batteries. Amp-hours is commonly abbreviated as Ah or, less accurately, as A. For example: 500 mAh (500 milliamp-hours) or 3.5 Ah (3.5 amp-hours, or 3500 milliamp-hours).
Very occasionally, and for some applications, batteries may be rated in watts, though this is an imperfect measure. Technically, wattage is calculated at voltage times current, or V * I (I stands for current—for the time being, don’t worry about why; that’s just the way it is). So a battery operating at a nominal 12 volts, delivering 2 amp-hours, is rated at 24 watts.
UNDERSTANDING INTERNAL RESISTANCE
Batteries, like humans fighting the Borg, resist—in this case, they resist giving up their charge. This phenomenon is called internal resistance; the higher it is, the less current a battery can deliver at any moment. The internal resistance of a battery determines the maximum rate at which current can be drawn from the cells.
![]() Alkaline and lithium-ion batteries have a high internal resistance. These can still deliver current, but they cannot “dump” all of it within a very short period. Think of them as long-distance runners.
Alkaline and lithium-ion batteries have a high internal resistance. These can still deliver current, but they cannot “dump” all of it within a very short period. Think of them as long-distance runners.
![]() Lead-acid, NiCd, and NiMH batteries have low internal resistance. If necessary, these batteries will empty their charge within minutes, depending on the demands of the load. They’re the sprinters of the track-and-field team.
Lead-acid, NiCd, and NiMH batteries have low internal resistance. If necessary, these batteries will empty their charge within minutes, depending on the demands of the load. They’re the sprinters of the track-and-field team.
Bigger batteries have more surface area inside them, which also affects internal resistance. That’s why big batteries of any given type can power higher loads.
For most robotics applications, internal resistance isn’t a major issue. When it really matters is for those tasks where extreme (and short-lived) current draw may be required. Examples are combat robots and the battery used to power the electric propeller on a self-guided drone plane.
![]()
Rapid, high current discharge of any battery can result in the cell overheating, fire, even explosion! That’s why you never want to intentionally short out the terminals of a battery. This causes the battery to disgorge all of its current as quickly as it can.
UNDERSTANDING BATTERY RECHARGE RATE
Most batteries are recharged more slowly than they are discharged. A good rule of thumb when recharging any battery is to limit the recharging level to one-half to one-tenth the amp-hour rating of the cell. For a 5-amp-hour battery, a safe recharge level might be 500 to 2500 milliamps.
Limiting current is extremely important when recharging NiCd and NiMH cells, which can be permanently damaged if charged too quickly. Lead-acid batteries can take an occasional “fast-charge.” However, repeated quick-charging will lessen the life of the battery.
The recharge period, the number of hours the battery is recharged, varies depending on the type of cell. A recharge interval of 2 to 10 times the discharge rate is recommended.
Recharging Batteries
Nickel metal hydride, rechargeable alkalines, and rechargeable lithium-ion batteries all require special rechargers. Avoid substituting the wrong charger for the battery type you are using, or you run the risk of damaging the charger and/or the battery—and perhaps causing a fire.
Batteries are recharged by applying voltage and current to their power terminals. Exactly how much voltage, and how much current, depends on the type of battery. Some general tips and observations:
![]() Most lead-acid batteries can be recharged using a simple 200-to 1000-mA battery charger. The charger can even be a DC adapter for a video game, as long as the output voltage of the DC adapter is slightly higher than the voltage of the battery. Remove the battery from the recharger after 24 hours.
Most lead-acid batteries can be recharged using a simple 200-to 1000-mA battery charger. The charger can even be a DC adapter for a video game, as long as the output voltage of the DC adapter is slightly higher than the voltage of the battery. Remove the battery from the recharger after 24 hours.
![]() Standard NiCd batteries like to be recharged slowly, typically with currents under 100 or 200 mA. Use a charger that supplies too much current, and you will destroy the cell.
Standard NiCd batteries like to be recharged slowly, typically with currents under 100 or 200 mA. Use a charger that supplies too much current, and you will destroy the cell.
![]() Rechargeable alkaline, NiMH, and lithium-ion must use a battery charger designed for them. High-capacity NiCd batteries can be charged at higher rates, and there are rechargers designed especially for them.
Rechargeable alkaline, NiMH, and lithium-ion must use a battery charger designed for them. High-capacity NiCd batteries can be charged at higher rates, and there are rechargers designed especially for them.
![]() Always observe polarity when recharging batteries. Inserting the cells backward in the recharger will destroy the batteries and possibly damage the recharger.
Always observe polarity when recharging batteries. Inserting the cells backward in the recharger will destroy the batteries and possibly damage the recharger.
![]() Lithium-ion batteries can catch fire if they are incorrectly recharged. Only use a recharger specifically designed for the cell or battery pack you are using.
Lithium-ion batteries can catch fire if they are incorrectly recharged. Only use a recharger specifically designed for the cell or battery pack you are using.
Robot Batteries at a Glance
As you’ve seen, batteries can be rechargeable or nonrechargeable. And different battery types also vary by the volts per cell. Table 18-1 shows, in a nutshell, the common battery types most often used in robotics, the nominal voltage they deliver per cell (when fully charged), and other selection criteria.
Common Battery Sizes
Battery sizes have been standardized for decades (see Figure 18-7), though most consumers are familiar with just a few of the more common types: N, AAA, AA, C, A, and 9-volt. There are many other “in-between” sizes as well.
For the most part, the size of the battery directly affects its capacity—assuming the same types of batteries are compared. For example, because a C battery provides roughly double the internal area of an AA battery, it stands to reason that the capacity of a C battery is about twice that of the AA cell. (In actual practice, size versus capacity is more complicated than this, but it’ll do for a basic comparison.)
Table 18-1 Batteries and Their Ratings
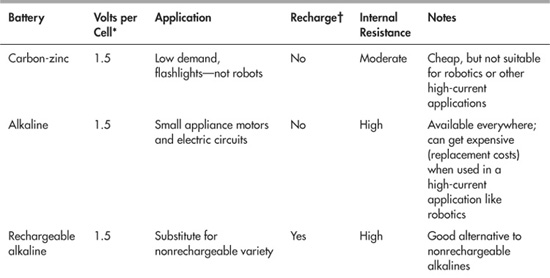
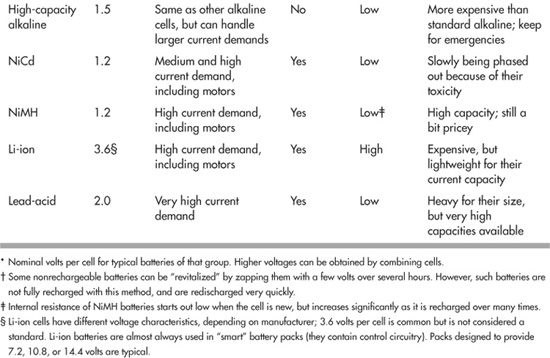
Figure 18-7 Comparison of consumer battery sizes, from N to D cell, plus 9-volt.
![]()
There’s more about batteries, battery sizes, and selection on the RBB Online Support site (see Appendix A for more details).
Increasing Battery Ratings
Through the magic of electricity, you can get higher voltages and current by connecting two or more cells together. Increasing volts or current depends on how you wire the batteries together:

Take note: When you connect cells together, not all cells may be discharged or recharged at the same rate. This is particularly true if you combine two half-used batteries with two new ones. The new ones will do the lion’s share of the work and won’t last as long. Therefore, you should always replace or recharge all the cells at once. Similarly, if one or more of the cells in a battery pack are permanently damaged and can’t deliver or take on a charge like the others, you should replace it.